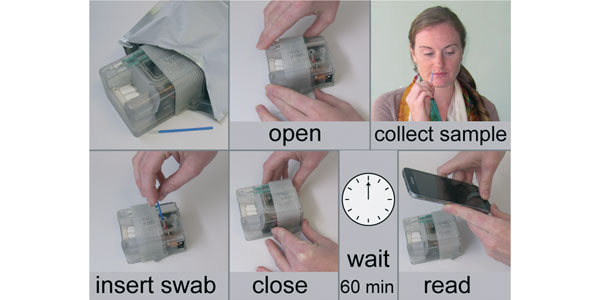
Image: The MAD NAAT user experience: user steps required to operate the MAD NAAT prototype from upper left to lower right. The sample collection may be performed by the user or a healthcare provider, depending on the setting. Device closure is the single activation step required; all other assay steps are internal and automated. Results can be interpreted by eye or recorded and quantitatively analyzed with a cell phone camera.
Abstract
The prototype demonstrated here is the first fully integrated sample-to-result diagnostic platform for performing nucleic acid amplification tests that requires no permanent instrument or manual sample processing. The multiplexable autonomous disposable nucleic acid amplification test (MAD NAAT) is based on two-dimensional paper networks, which enable sensitive chemical detection normally reserved for laboratories to be carried out anywhere by untrained users. All reagents are stored dry in the disposable test device and are rehydrated by stored buffer. The paper network is physically multiplexed to allow independent isothermal amplification of multiple targets; each amplification reaction is also chemically multiplexed with an internal amplification control. The total test time is less than one hour. The MAD NAAT prototype was used to characterize a set of human nasal swab specimens pre-screened for methicillin-resistant Staphylococcus aureus (MRSA) bacteria. With qPCR as the quantitative reference method, the lowest input copy number in the range where the MAD NAAT prototype consistently detected MRSA in these specimens was ?5 × 103 genomic copies (?600 genomic copies per biplexed amplification reaction).