New study presents the first biomaterial designed for vascular grafts with tuned mechanical properties and a precision-engineered porous structure optimized for healing.
Coronary artery disease and stroke affect about 19 million Americans, and vascular disease is responsible for the majority of amputations. To treat and prevent these conditions, about 1.4 million vascular grafts are used each year to replace diseased blood vessels. Also, about 15% of patients on hemodialysis receive vascular grafts for blood access.

Buddy Ratner is the Michael L. & Myrna Darland Endowed Chair in Technology Commercialization.
Today, there are no commercially available small-diameter vascular grafts. Buddy Ratner, UW professor of bioengineering and of chemical engineering, estimates that worldwide, more than a million leg amputations a year might be prevented if such a device existed, and coronary artery surgery might be greatly simplified for millions more patients. Medium-diameter grafts, made of stiff materials, have a 50 percent failure rate in the first year when used for dialysis access, often stemming from a foreign body reaction, which results in scar tissue buildup and a blocked blood vessel.
Researchers at the University of Washington have created a flexible graft engineered to match the mechanical properties of native blood vessels and heal in place like a natural artery. Because it “looks” more like a natural blood vessel to the body, the immune system reacts favorably, leading to more healing and less scar tissue.
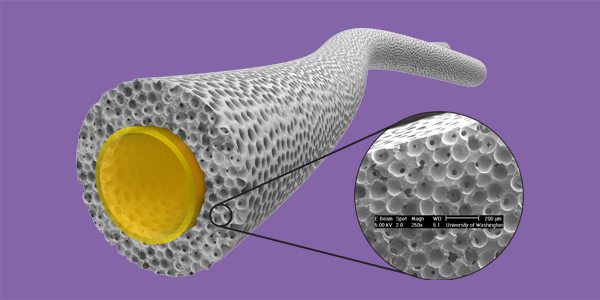
Led by the Ratner lab, collaborators at UW developed a biostable, crosslinked polyurethane material and used it to build scaffolds with precision-engineered tiny, interconnected pores 40 micrometers in diameter – roughly half the width of a human hair. They made scaffolds with varying pore sizes and tested them in mice for three weeks. Their results show that the 40 micrometer porous scaffolds generated the highest level of new blood vessel formation, creation of new tissue, and the least severe foreign body capsule reaction.
The researchers published their results in October in Biomaterials.
“This study presents the first biomaterial with tuned mechanical properties and a precision engineered porous structure optimized for healing,” the researchers wrote. They added that their scaffolds should be “ideal materials for future pro-healing vascular grafts and in-situ vascular engineering.”
Le Zhen, a postdoctoral researcher in the Buddy Ratner lab, is the lead author on the paper. Co-authors are Sharon A. Creason, Felix I. Simonovsky, Jessica M. Snyder, Sarah L. Lindhartsen, Marvin M. Mecwan, Brian W. Johnson, Jonathan Himmelfarb and Buddy D. Ratner. The study was funded with a grant from the Northwest Kidney Centers and a gift from Paula and Steve Reynolds to the Center for Dialysis Innovation (CDI).