UW Bioengineering researchers identify ways of turning off the function of a blood protein under conditions that may encourage dangerous clots.
When you get a cut or other internal or external injury – any time a blood vessel is damaged – a protein known as von Willebrand factor (VWF) goes into action. It binds to blood platelets and attaches them to the injury site, causing coagulation to begin. The coagulating blood thickens, closing the wound with a clot and giving it the chance to heal.
Not all clots are welcome, however. The development of clots in veins and arteries, called thrombosis, can block blood flow and cause pain or swelling. The clots can also break loose and flow to the heart or lungs. This can lead to pulmonary embolism, stroke, or heart attack.
“Drugs that are currently used to treat thrombosis – blood thinners like warfarin or Eliquis – create the risk of excessive bleeding as a side effect,” Gianluca Interlandi, the principal investigator on the studies, said. “We’re asking: Is it possible to design a therapeutic that blocks the formation of a dangerous clot without affecting the typical, beneficial clotting response that we want in case of injury?”
Take, for instance, areas in the body that are inflamed. Leukocytes, a type of immune cells, release hydrogen peroxide, which is converted to hypochlorous acid. This acid oxidizes methionine residues in blood proteins modifying their structure and behavior. In the case of VWF, oxidation of methionine residues increases the likelihood that it will produce dangerous clots. If Interlandi and his collaborators can identify drugs that inhibit only oxidized VWF, they could reduce the risk of dangerous clots. At the same time, they would leave VWF that is not oxidized unimpacted and available for hemostasis, which is the healthy clotting needed to stop blood loss from injury.
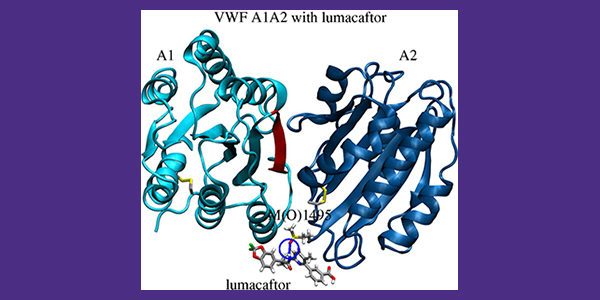
Using computational modeling, they identified a ligand that binds to a particular spot on oxidized VWF molecules. The ligand prevents the oxidized VWF from subsequently binding to blood platelets and encouraging excessive clotting. In a related study, the team also developed an enzyme-linked immunosorbent assay to test the impact of existing drugs, such as the one identified in the computational studies, on the ability of VWF to bind to platelets. These tests showed that the molecular structure of the drug lumacaftor, which is currently used to treat cystic fibrosis, can be used as the basis to design an inhibitor of oxidized VWF under inflammatory conditions.
These findings were published in Proteins in 2024 and in a preprint released in August 2025.