FROM DISCOVERY TO APPROVAL
Through the PharBE program, students gain a clear understanding of the entire drug and device development process, from drug discovery and design to clinical trial phases, market analysis and comparing strategies to get drugs approved. Program courses take a dive into the individual steps in the process, from drug classes to regulatory issues, and a capstone course ties it all together.
“Having detailed knowledge of the overall process allows graduates to participate fully in drug development, understand where they can contribute, and see where their contribution fits into the product life cycle,” says Roberta Wong, co-director of the PharBE program and associate assistant professor in Bioengineering.
WHO IS THE PROGRAM FOR?
The PharBE program is tailored for people in the pharmaceutical industry, such as bench scientists who want to advance their careers or move into another aspect of the biotech industry, or manufacturing associates who want to have more knowledge of drug design and development.
Offered entirely online since 2017 and designed for working professionals worldwide, the part-time program can be completed in less than two years.
LEARN FROM EXPERTS IN THE FIELD

Robbie Wong

Edward Kelly

Patrick Stayton
The PharBE program gives students direct access to faculty who have first-hand experiences with the drug development process, both in academia and industry. Roberta Wong, BioE associate professor, is a Seattle-based pharmaceutical consultant who was senior project manager at Amgen, director of corporate planning at Abraxis Bioscience, and director of research at Agensys. She co-directs the program with two other UW professors. Edward Kelly, is UW associate professor of pharmaceutics, whose research is focused on using “organs on chips” or microphysiological systems (MPS) to model human diseases. Kelly managed the Preclinical Bioanalytics group at Targeted Genetics Corporation before joining UW’s faculty. Patrick Stayton is Distinguished Career Professor of Bioengineering and director of the Molecular Engineering and Sciences Institute, who has developed therapies and co-founded two companies to move those technologies into market.
“We were exposed to experts in the field on a regular basis,” says alumna Kristen Fetchko, clinical study manager at CytoSorbents. “Not only did this allow for some great lectures, but it allowed us to ask real-world questions and receive answers that you can’t get from a textbook or a pre-recorded lecture.”
The program also features upper-level industry experts from diverse backgrounds, with hands-on knowledge and experience taking drug candidates from concept to commercial market. As guest instructors they offer students a breadth of exposure across the industry.
“Most programs, at least for master’s, are very rare in terms of the breadth that the UW PharBE program offered, from clinical to commercial to process development,” says alumnus Jeffrey Chu, Manager, Biologics Technical Development at Horizon.
The students also learn from each other. The program draws a broad mix of students, from those right out of undergraduate programs to bench scientists, lawyers, medical professionals and those with MBAs. Their diverse experiences create a rich learning environment. Students are encouraged to share insights from their daily work, including the manufacturing process, challenges in animal studies or experiments with drug candidates.
CURRICULUM FOR THE REAL WORLD
Students complete a core curriculum in Basic Biosciences, choose two electives and take six credits of Departmental Seminars, which feature industry speakers from a range of biotech areas. Finally, each student completes a capstone project, in which they develop a life cycle strategy plan for a real-life drug currently in phase 2 clinical trials. In the team project, teams of two or more develop a go/no-go analysis for a different drug or device to determine whether a product has enough merit to finish drug development with the goal of being submitted for FDA review and approval.
CONVENIENT, BUT CONNECTED
As one of the first all-online PharBE programs, UW BioE developed ways to make online learning engaging and effective, and honed them before the coronavirus pandemic struck.
Live class discussions happen once a week in the evening, and students watch pre-recorded sessions at their convenience. The program uses Zoom video conferencing, so in addition to a main screen with slides, video or a whiteboard, the instructor and all students can see and hear each other via thumbnail video feeds, and use the live chat feature. In addition to drawing on the whiteboard and creative videos, some instructors hold live oral exams. All live lectures are also recorded, so students can watch the recordings as often as they like.
Wong says some people are concerned that in an online class, you are anonymous, you don’t feel part of a group, and that you may not learn as much because of it. She points out that students in 2019’s graduating class “all felt really connected,” even though they were spread out across the country from upstate New York, Boston, San Diego, Chicago and one in Seattle. Out of 15 that graduated, 12 of them traveled to the graduation ceremony in Seattle. “You’re talking about a pretty big commitment from people feeling connected to the program,” she says. She sought her students out as they gathered on campus before the ceremony. Although students were worried they wouldn’t recognize their peers, they said everyone looks just the same in person as their online video image.
They were chatting a mile a minute when they saw each other, even though for most of them it was the first time they had seen each other in person – Robbie Wong
Thanks to her pre-pandemic travel schedule in 2019, Wong was able to personally meet every student who graduated in that class. She also does one-on-one videos with her students to help her get to know them as individuals.
In addition to recorded and live online classes, students also interact with each other through team projects that promote communication and colleague-building skills. Students get practice presenting, listening to their classmates, accepting and offering feedback, dissecting strategy and thinking critically.
“Robbie [Wong] really emphasized team engagement, classroom dynamics and working together, and that was a huge part in getting to know people,” alumnus Jeffrey Chu of Horizon Therapeutics says.
Offering the program entirely online gives students flexibility to fully participate regardless of where they live, how much they may travel for work in the future or even if their company moves,” Wong says.
DRUG DEVELOPMENT IN THE BUSINESS CONTEXT
The hallmark feature of the program is the individual capstone project. Each student is assigned a drug that is currently in phase 2 development, meaning that it has shown some results that it works. Then each student designs a phase 3 clinical trial and a development strategy for successfully earning FDA approval and getting the drug on the market. During the course, students also develop a strategic plan for publishing studies and a SWOT (strengths, weaknesses, opportunities and threats) analysis aimed at marketing it throughout its patent life.
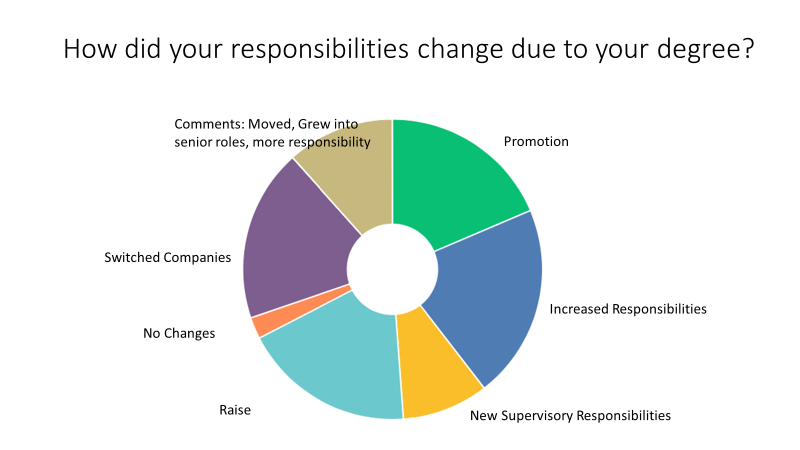
Respondents to a recent survey of UW PharBE program alumni shows a large majority moved to a new position or took on more responsibility.
“The capstone course is the most unique aspect of the program,” Wong says. Other programs go over nuts and bolts – what she calls “a cookbook method” of drug development – but the UW BioE program provides some real-life experience in what it would be like to do this job for a company.
“UW PharBE students have to analyze the market, look at the competition and figure out how they can position their product to be potentially a leader in the treatment of that particular disease. I haven’t seen any other program that provides this experience to students,” Wong says.
Wong says many of her students come from a laboratory-based background, working at a stage before drugs are tested in humans or in process development and manufacturing.
She provides an understanding of what happens when drugs are tested in people, and how the work they’re doing in the lab and manufacturing impacts human trials.
“I also explain how business decisions could affect continued development of a drug product to commercialization,” Wong says.
Notably, the capstone course features a risk-benefit analysis, given the expense of developing and winning marketing approval for a drug, which can exceed $2.5 billion. “One of the most important questions arising in the last 5-10 years is: does the development cost for the drug, testing and monitoring, give you a product that will advance the treatment of disease?” Wong says. “Do you get a better quality of life? Do you extend life, as in the case of cancer or other life-threatening diseases such as hepatitis? And what is that worth – does it cost $100,000 to get four more months of life, or is it a $100,000 investment and we get 20 more years of life and save 10 hospitalizations and their potential complications?”
The course includes a discussion on the reality of potential drug adoption or use in patients. In drug development, it’s not just about finding a drug that works – people must be able to afford it.
Insurance companies and clinicians need strong evidence of a drug’s effectiveness, otherwise it might not get used. Many people may not have the resources to pay for expensive drugs without an insurance company’s help. “New drugs not only need strong efficacy that is great improvement over current treatment options, they need to also be affordable, so we don’t waste a drug that could help people but is not accessible to those who most need it,” Wong says. Students often name class discussions about business decisions as one of the highlights of the program, and that knowledge really helps students participate in cross-function teams in their jobs, she adds.
The capstone class is critically important, Wong says, especially as more insurance policies cover fewer conditions and drugs. “You have to be aware of the expense of drug treatment, and the benefit it provides to patients,” she says. “Cost does matter.”
In addition to understanding how drugs are developed, graduates review how diseases are managed and look at how decisions are made in selecting which drugs are used and not used, Wong says.
Details about the program and answers to frequently asked admissions questions are available on the PharBE Admissions FAQ page.

