Bioengineering Distinguished Term Professor
Director, Molecular Engineering and Sciences Institute
stayton@uw.edu
Phone: (206) 685-8148
Office: Foege N530L

Patrick S. Stayton
Molecular engineering
Molecular imaging
Targeted drug delivery
“Smart” Biotechnology and Nanotechnology
We are developing new biohybrid molecular materials designed to “talk” and “listen”. These “smart” materials are designed for applications in the drug delivery and bioanalytical fields. The drug delivery group is working to develop functional and pH-responsive polymeric carriers for biomolecular therapeutics and vaccines, e.g. proteins, antisense oligonucleotides, RNA interference or silencing RNA, and DNA plasmids. Our challenge is to develop carriers that mimic the ability of viruses and pathogens to deliver macromolecules to specific intracellular compartments, while avoiding their immunogenicity and toxicity.
The bioanalytical group is working to develop smart polymer-protein conjugates, smart polymer-DNA conjugates, and smart polymer-bead conjugates as responsive molecular componentry for diagnostics, lab assays, biochips and arrays, and for upstream processing of complex fluids such as blood. We have a focus on point-of-care diagnostics, including with the Distributed Diagnosis and Home Healthcare group and in diagnostics for resource-poor settings, e.g. diagnostics for Africa.
Biomaterials and Tissue Engineering
We are working in collaboration with the Biomaterials and Tissue Engineering group to develop a better understanding of the mechanisms by which materials can be engineered to control cellular responses. A primary focus is on controlling the foreign body reaction to biomaterials. To accomplish this goal, mechanistic studies of macrophage activation on biomaterials are combined with the development of controlled release systems for anti-inflammatory delivery. For the fundamental studies, signaling pathway analysis is being conducted using gene expression profiling and proteomics to identify key molecular targets for controlling cell response to biomaterials. The delivery arm of the project is utilizing new delivery systems for antisense and RNA interference therapeutics that inactivate key macrophage signaling targets or inflammatory cytokines.
Another focus area is controlling vascularization around tissue engineering matrices and tissue regeneration materials. Our work is centered on protein and nucleic acid delivery from hydrogel coatings and matrices. Similar strategies for protein growth factor and nucleic acid delivery from hydrogel matrices are being used to promote hard tissue regeneration for craniofacial and dental applications.
Molecular Recognition Studies
Our applied bioengineering projects benefit from connections to more fundamental biophysical studies of molecular recognition in biology. We have two primary projects, the first in the area of biomineralization. This research is directed toward elucidating the fundamental design principles used by nature to engineer bone and teeth. We are investigating the molecular mechanisms used by proteins to control the hierarchical structure of biological calcium composites such as hydroxyapatite and calcium oxalate. These studies connect to our hard tissue regeneration applications.
The second project studies the detailed molecular mechanisms by which proteins regulate small molecule recognition. We are using a combination of site-directed mutagenesis, biophysical characterization, and high-resolution structural characterization to elucidate how proteins generate high-affinity for small molecule ligands. These studies may illuminate design principles for drug design, where high affinity is generally the desired goal. The central project is directed toward determining the structure-function relationships responsible for high-affinity and slow off-rates in the model streptavidin-biotin system. These studies connect to more applied protein engineering efforts with streptavidin, which is a widely utilized protein in diagnostics and bioanalytical technologies.
Targeted Drug Delivery
Targeting of therapeutics and imaging agents to specific sites in the body is an important aspect of drug delivery systems. We are using genetic engineering techniques to design antibody and streptavidin systems for improved performance in targeted drug delivery systems. Our model systems are designed for in vivo delivery of therapeutics and radionucleotides to carcinomas such as B-cell lymphoma.
Controlled Release Society, CRS-Cygnus Recognition Award
Hunter Visiting Professor, Clemson University
Minority Science Engineering Program, Honorary Award
D Roy, B Ghosn, EH Song, DM Ratner, PS Stayton. Polymer–trimannoside conjugates via a combination of RAFT and thiol–ene chemistry Polymer Chemistry 2013.
Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH- Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano. 2013 May 28;7(5):3912-25. doi: 10.1021/nn305466z. Epub 2013 Apr 30. PubMed PMID: 23590591
Nash MA, Waitumbi JN, Hoffman AS, Yager P, Stayton PS. Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system. ACS Nano. 2012 Aug 28;6(8):6776-85. doi: 10.1021/nn3015008. Epub 2012 Jul 24. PubMed PMID:22804625
Langmuir. 2013 May 7;29(18):5388-93. doi: 10.1021/la400347r. A Photoinduced Nanoparticle Separation in Microchannels via pH-Sensitive Surface Traps. Ebara M, Hoffman JM, Hoffman AS, Stayton PS, Lai JJ.
Ebara M, Hoffman JM, Hoffman AS, Stayton PS, Lai JJ. Langmuir. A Photoinduced Nanoparticle Separation in Microchannels via pH-Sensitive Surface Traps.2013 May 7;29(18):5388-93. doi: 10.1021/la400347r. PubMed PMID: 23581256
Hoffman JM, Ebara M, Lai JJ, Hoffman AS, Folch A, Stayton PS. A helical flow, circular microreactor for separating and enriching “smart” polymer-antibody capture reagents. Lab Chip. 2010. 10(22):3130-8. PubMed PMID: 20882219.
Nash MA, Yager P, Hoffman AS, Stayton PS. Mixed Stimuli-Responsive Magnetic and Gold Nanoparticle System for Rapid Purification, Enrichment, and Detection of Biomarkers. Bioconjug Chem. 2010. 21(12): 2197-2204. PubMed PMID: 21070026
Golden AL, Battrell CF, Pennell S, Hoffman AS, Lai JJ, Stayton PS. Simple fluidic system for purifying and concentrating diagnostic biomarkers using stimuli-responsive antibody conjugates and membranes. Bioconjug Chem. 2010. 21(10):1820-6. PubMed Central PMCID: PMC2958215.
Henry SM, Convertine AJ, Benoit DS, Hoffman AS, Stayton PS. End-functionalized polymers and junction-functionalized diblock copolymers via RAFT chain extension with maleimido monomers. Bioconjug Chem. 2009. 20(6):1122-8. PubMed PMID: 19480416; PubMed Central PMCID: PMC2884002.
11. Nash MA, Lai JJ, Hoffman AS, Yager P, Stayton PS. “Smart” diblock copolymers as templates for magnetic-core gold-shell nanoparticle synthesis. Nano Lett. 2010.10(1):85-91. PubMed PMID: 20017498; PubMed Central PMCID: PMC2806508.
Lai JJ, Nelson KE, Nash MA, Hoffman AS, Yager P, Stayton PS. Dynamic bioprocessing and microfluidic transport control with smart magnetic nanoparticles in laminar-flow devices. Lab Chip. 2009 Jul 21;9(14):1997-2002. PubMed PMID: 19568666; PubMed Central PMCID: PMC2902376.
Lai JJ, Hoffman JM, Ebara M, Hoffman AS, Estournès C, Wattiaux A, Stayton PS. Dual magnetic-/temperature-responsive nanoparticles for microfluidic separations and assays. Langmuir. 2007 Jun 19;23(13):7385-91. Epub 2007 May 16. PubMed PMID: 17503854.
Narain R, Gonzales M, Hoffman AS, Stayton PS, Krishnan KM. Synthesis of monodisperse biotinylated p(NIPAAm)-coated iron oxide magnetic nanoparticles and their bioconjugation to streptavidin. Langmuir. 2007 May 22;23(11):6299-304. Epub 2007 Apr 24. PubMed PMID: 17451262.
Kulkarni S, Schilli C, Grin B, Müller AH, Hoffman AS, Stayton PS. Controlling the aggregation of conjugates of streptavidin with smart block copolymers prepared via the RAFT copolymerization technique. Biomacromolecules. 2006 Oct;7(10):2736-41. PubMed PMID: 17025347.
Ebara M, Hoffman JM, Hoffman AS, Stayton PS. Switchable surface traps for injectable bead-based chromatography in PDMS microfluidic channels. Lab Chip. 2006 Jul;6(7):843-8. Epub 2006 May 5. PubMed PMID: 16804587.
In the News
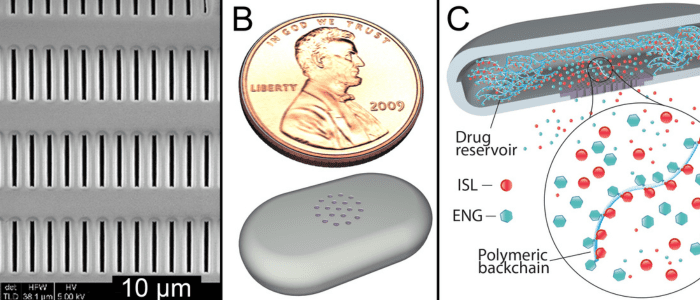
Researchers are developing an implantable device that could prevent pregnancy and HIV for two years
2023-09-12T09:14:13-07:00September 12th, 2023|
UW Bioengineers pivot to develop coronavirus solutions
2022-08-01T14:43:01-07:00July 9th, 2020|
Online Master of Pharmaceutical Bioengineering prepares working professionals to drive drug discovery, development
2022-07-13T14:03:17-07:00December 13th, 2016|
UW, Academia Sinica researchers develop “drug capture system” to treat limb ischemia
2020-10-26T08:29:48-07:00December 6th, 2016|
Patrick Stayton, Valerie Daggett to present 2016-17 Science in Medicine Lectures
2021-01-21T06:04:29-08:00September 30th, 2016|
Patrick Stayton named Distinguished Career Professor of Bioengineering
2020-10-26T08:30:02-07:00March 14th, 2016|