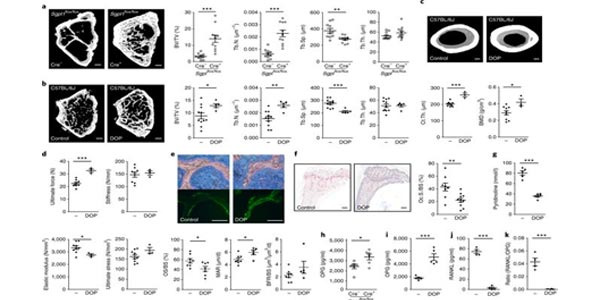
Image: Deficiency or inhibition of S1P lyase results in higher bone mass and strength, altered OC activity and increased plasma OPG. View full image details.
Targeting sphingosine-1-phosphate lyase as an anabolic therapy for bone loss
Sarah Weske, Mithila Vaidya, Alina Reese, Karin von Wnuck Lipinski, Petra Keul, Julia K Bayer, Jens W Fischer, Ulrich Flögel, Jens Nelsen, Matthias Epple, Marta Scatena, Edzard Schwedhelm, Marcus Dörr, Henry Völzke, Eileen Moritz, Anke Hannemann, Bernhard H Rauch, Markus H Gräler, Gerd Heusch and Bodo Levkau.
Nature Medicine. Volume 24, pages 667–678 (2018).
Abstract
Sphingosine-1-phosphate (S1P) signaling influences bone metabolism, but its therapeutic potential in bone disorders has remained unexplored. We show that raising S1P levels in adult mice through conditionally deleting or pharmacologically inhibiting S1P lyase, the sole enzyme responsible for irreversibly degrading S1P, markedly increased bone formation, mass and strength and substantially decreased white adipose tissue. S1P signaling through S1P2 potently stimulated osteoblastogenesis at the expense of adipogenesis by inversely regulating osterix and PPAR-?, and it simultaneously inhibited osteoclastogenesis by inducing osteoprotegerin through newly discovered p38–GSK3?–?-catenin and WNT5A–LRP5 pathways. Accordingly, S1P2-deficient mice were osteopenic and obese. In ovariectomy-induced osteopenia, S1P lyase inhibition was as effective as intermittent parathyroid hormone (iPTH) treatment in increasing bone mass and was superior to iPTH in enhancing bone strength. Furthermore, lyase inhibition in mice successfully corrected severe genetic osteoporosis caused by osteoprotegerin deficiency. Human data from 4,091 participants of the SHIP-Trend population-based study revealed a positive association between serum levels of S1P and bone formation markers, but not resorption markers. Furthermore, serum S1P levels were positively associated with serum calcium , negatively with PTH , and curvilinearly with body mass index. Bone stiffness, as determined through quantitative ultrasound, was inversely related to levels of both S1P and the bone formation marker PINP, suggesting that S1P stimulates osteoanabolic activity to counteract decreasing bone quality. S1P-based drugs should be considered as a promising therapeutic avenue for the treatment of osteoporotic diseases.