CURRICULUM FOR THE REAL WORLD
Students complete a core curriculum in Basic Biosciences, choose two electives and take six credits of Departmental Seminars, which feature industry speakers from a range of biotech areas. Finally, each student completes a capstone project, in which they develop a life cycle strategy plan for a real-life drug currently in phase 2 clinical trials. In the team project, teams of two or more develop a go/no-go analysis for a different drug or device to determine whether a product has enough merit to finish drug development with the goal of being submitted for FDA review and approval.
Master of Pharmaceutical Bioengineering – Curriculum
Basic Bioscience Block
This block is also a UW Professional and Continuing Education Certificate Program.
- Investigates the core disciplines involved in drug design, discovery and clinical drug development.
- Examines the fundamental molecular processes in organisms, the molecular structure-function relationship, and discusses how diseases are treated.
- Discusses the manufacturing challenges for drug and vaccine development.
- Explains the pharmaceutical industry structure and how a new drug is developed and brought to market.
- Highlights business and science considerations.
Course Descriptions:
Each course has one live evening lecture and one pre-recorded lecture per week. These courses are open to PHARBE Degree or Basic Biosciences Certificate.
| Autumn Quarter | PHARBE 500 – Molecular and Cellular Biology for Pharmaceutical Bioengineering I, 4 Credits Fundamental molecular processes that occur in cells using cancer as a platform. Genome expression, epigenetics, microRNA, intracellular signaling, control of cell cycle, and cell differentiation and metastasis. Reproductive system molecular biology, immunology, the molecular biology of the musculoskeletal system and the human biome and disease are also included. Use of online literature/media resources and bioinformatics tools are integrated into course. |
| Winter Quarter | PHARBE 505– Pathophysiology for Pharmaceutical Bioengineering, 4 Credits Introduction to pathophysiology of human systems, including nervous, respiratory, cardiovascular, gastrointestinal, endocrine, renal, and urinary systems. Medical bioengineering innovations and case histories are integrated into the course, along with online literature/media resources and bioinformatics tools. |
| Spring Quarter | PHARBE 502 – Pharmaceutics I, 4 Credits This course covers the drug discovery and design process for both small molecules and biologics from discovery stage through Phase 1 Trial design and IND Filing. Topics include science, logistics, and regulatory environment of preclinical (pharmacology, PKDM, toxicology, CMC) and early clinical development. |
| Summer Quarter | PHARBE 503 – Pharmaceutics II, 5 Credits This course focuses on Clinical Drug Development which is the most expensive part of drug development. After reviewing Phase 1 trial design we discuss Phase II and III Trial Design. Topics include clinical study design, safety, regulatory, CMC, toxicology and statistics. An overview of New Drug Applications (NDAs) and Biologics License Application (BLAs) of a variety of drug classes, and proteins along with Advisory Committee (AC) meetings is also included. Device development is also discussed. Prerequisite: PHARBE 502 or permission of instructor. |
Choose one or both electives in Autumn and Winter quarters.
You must take at least one elective in each quarter.
| PHARBE 521 – Drug Discovery and Design Autumn Quarter, 5 Credits General principles and current approaches involved in modern drug discovery and development. Specific aspects of human biology and disease, case studies in discovery, and the evolution of how these topics have merged. Novel drug discovery techniques and emerging non-standard therapeutics and the history of drugs and drug discovery. |
AND/OR | PHARBE 510 – Applied Pharmacokinetics Autumn Quarter, 5 Credits Basic principles of pharmacokinetics and their application to the clinical setting, including: single-dose intravenous and oral kinetics, multiple dosing, nonlinear pharmacokinetics, metabolite kinetics, pharmacogenetics, and the role of disease in drug clearance and dose requirements, and kinetics of drug-drug interactions. |
| PHARBE 522 – Molecular Targets and Drug Classes
Winter Quarter, 5 Credits Discussion of key compounds and structural and physical properties governing mechanism of action. Design of therapeutics by class to maximize efficacy while reducing toxicities. Compounds may include small-molecules, peptides, proteins, nucleic acids, lipids, and vaccines. |
AND/OR | PHARBE 511 – Process Development
Winter Quarter, 5 Credits Principles involved in designing and optimizing production processes for various therapeutic agents including small molecules, proteins, and cell and gene therapy products. Technologies and methods used in the production of therapeutics and discussion of the interdependence of upstream, downstream, analytical, formulation and drug delivery technologies. |
PHARBE 513 – Clinical Development
2nd year – Spring Quarter, 6 Credits
Evaluating clinical development strategies including comprehensive drug candidate life cycle plan. Covers clinical trial design, FDA and Institutional Review Boards, ethics, consents, safety, and quality. Discussing target product profiles, phase III study synopses, informed consents, and SWOT analyses. Students design and present their Phase III Trial Synopsis, debate go/no-go decisions for continuing development or recommending stopping development. The main project is the capstone: creating a life-cycle management plan for a drug over the life of the patent. Device development is also discussed and contrasted with drug development.
Most quarters students will also take seminars which typically meet once a week for one or two hours. All session are live and also recorded with recordings available to students during the quarter.
| 1st year – Winter | BIOEN 509 Departmental Seminar Series |
| 1st year Spring | BIOEN 509 Departmental Seminar Series |
| 1st year Summer | No Seminar |
| 2nd year autumn | PHARBE Seminar |
| 2nd year Winter | BIOEN 509 Departmental Seminar Series |
| 2nd year Spring | BIOEN 509 Departmental Seminar Series |
FROM DISCOVERY TO APPROVAL
Through the PharBE program, students gain a clear understanding of the entire drug and device development process, from drug discovery and design to clinical trial phases, market analysis and comparing strategies to get drugs approved. Program courses take a dive into the individual steps in the process, from drug classes to regulatory issues, and a capstone course ties it all together.
“Having detailed knowledge of the overall process allows graduates to participate fully in drug development, understand where they can contribute, and see where their contribution fits into the product life cycle,” says Roberta Wong, co-director of the PharBE program and associate assistant professor in Bioengineering.
WHO IS THE PROGRAM FOR?
The PharBE program is tailored for people in the pharmaceutical industry, such as bench scientists who want to advance their careers or move into another aspect of the biotech industry, or manufacturing associates who want to have more knowledge of drug design and development.
Offered entirely online since 2017 and designed for working professionals worldwide, the part-time program can be completed in less than two years.
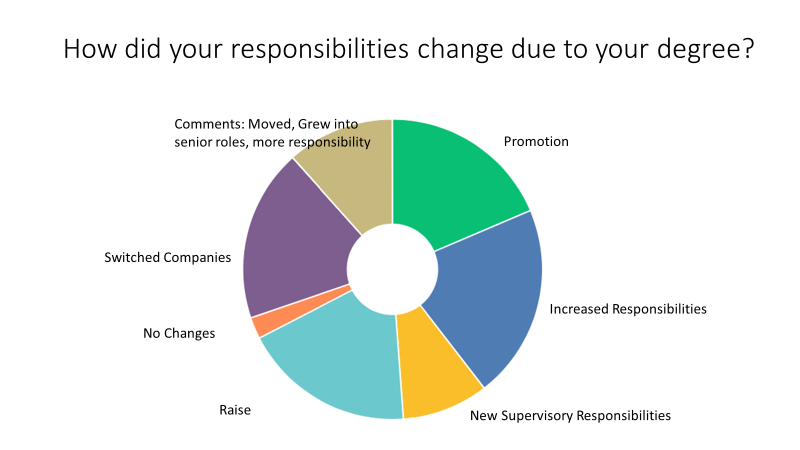
Respondents to a recent survey of UW PharBE program alumni shows a large majority moved to a new position or took on more responsibility.
DRUG DEVELOPMENT IN THE BUSINESS CONTEXT
The hallmark feature of the program is the individual capstone project. Each student is assigned a drug that is currently in phase 2 development, meaning that it has shown some results that it works. Then each student designs a phase 3 clinical trial and a development strategy for successfully earning FDA approval and getting the drug on the market. During the course, students also develop a strategic plan for publishing studies and a SWOT (strengths, weaknesses, opportunities and threats) analysis aimed at marketing it throughout its patent life.
“The capstone course is the most unique aspect of the program,” says Robbie Wong, PharBE program director. Other programs go over nuts and bolts – what she calls “a cookbook method” of drug development – but the UW BioE program provides some real-life experience in what it would be like to do this job for a company. I haven’t seen any other program that provides this experience to students,” Wong says.
Notably, the capstone course features a risk-benefit analysis, given the expense of developing and winning marketing approval for a drug, which can exceed $2.5 billion. The course includes a discussion on the reality of potential drug adoption or use in patients. In drug development, it’s not just about finding a drug that works – people must be able to afford it.
In addition to understanding how drugs are developed, graduates review how diseases are managed and look at how decisions are made in selecting which drugs are used and not used,
Details about the program and answers to frequently asked admissions questions are available on the PharBE Admissions FAQ page.


