Image: An illustration demonstrating agglutination of yeast cells tagged with red and blue fluorescent markers. Credit: Arjun Kakhar, UW BioE Ph.D. student.

David Younger
Beyond its humble role in baking bread and brewing beer, yeast has helped scientists establish modern understanding of cell biology and genetics. And now, yeast may lead the way to safer drugs.
A team of UW synthetic biologists led by recent BioE Ph.D. graduate David Younger developed a method to reprogram yeast’s mating habits to enable detailed drug interaction screening. Younger, fellow BioE Ph.D. graduate Stephanie Berger, and research advisors David Baker and Eric Klavins published their findings this fall in the Proceedings of the National Academy of Sciences. The work was also featured in an article published on The Conversation. By ushering in a yeast sexual revolution, Younger has created a sophisticated tool that can help scientists screen drugs for safety.
Yeast is often used in synthetic biology research as its cell replication cycle is fast – a new generation is created within 90 minutes – and its genes can be easily modified to accommodate human DNA, or genes that encode protein-based drugs.
Yeast strains are commonly altered via a technique called yeast surface display. With this method, genetic material is spliced into yeast cells, and the resulting protein appears on the cell’s surface. It is particularly useful in drug development as it allows researchers to easily see how a drug interacts with cellular targets.
A drug that binds where it’s supposed to could be an effective treatment, but might be deadly if it sticks to other proteins. Screening a drug for off-target interactions before clinical trials can save lives and enhance the chances of a drug’s success. However, since yeast surface display allows only one interaction to be studied at a time, using this method can delay R&D timelines and increase the cost of drug development.
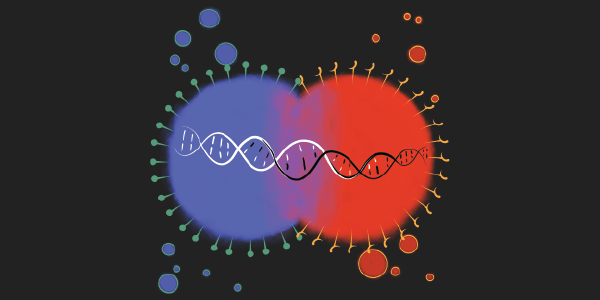
The benefits and shortfalls of using yeast surface display as a drug development platform inspired Younger to investigate ways it could be improved. He saw exploiting yeast’s mating mechanism, or agglutination, as a promising next step. Agglutination could extend the utility of yeast surface display by providing a way to see the interactions between two different types of modified cells.
To get started, Younger needed a way to precisely measure the efficiency of yeast mating, and to determine strong or weak interactions. He tagged yeast strains with blue and red fluorescent markers – one for each of the yeast mating types – and deleted the yeast’s agglutination proteins. Using flow cytometry, he counted the number of purple cells resulting from successful mating.
With his assay, Younger aimed to replicate yeast’s natural mating efficiency. He first tried substituting the natural agglutination proteins with weaker foreign ones and found the yeast’s mating efficiency to be poor. However, he discovered that swapping in strongly interacting proteins increased it to 52% – close to the 60% level seen in unaltered yeast.
Younger now had a simple way to quantify yeast mating success. However, figuring out how to apply his method to examine the thousands of interactions needed for drug screening was key. The team created 1,400 distinct variants of XCDP07, an emerging cancer drug. XCDP07 halts cancer cell growth by binding to a specific cellular target – and it can be made useless if it binds the wrong targets.
The researchers developed a library containing the XCDP07 variants and 5 protein targets, establishing 7,000 possible interactions, and encoded each protein with a short DNA sequence “barcode.” They then replaced the yeast’s agglutination proteins with the drug variants and protein targets, each tagged with a unique barcode. By using next-generation sequencing, they were able to identify which versions of the drug bound with the intended target and the drug specificity.
Currently a UW CoMotion Innovation Fellow, Younger established a company, A-Alpha Bio, to bring his technology to market. Earlier this year, A-Alpha Bio received UW’s Science and Technology Showcase Grand Prize. Younger also teamed with BioE Ph.D. student Bob Lamm to present A-Alpha Bio at May’s UW Business Plan Competition, where they won the Best Technology/Innovation Prize. The team has received funding from CoMotion, and is currently seeking research and pharmaceutical company partners.