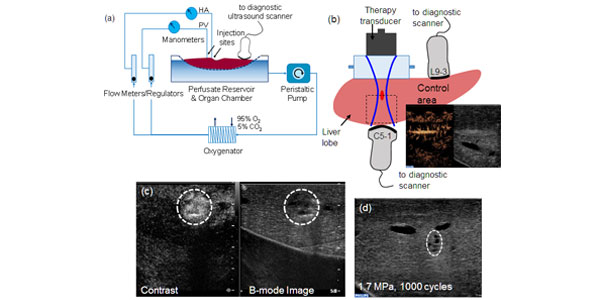
Image: (a) Schematic representation of the machine perfusion system. The livers were connected to the developed sub-normothermic machine perfusion system and sustained for up to 4 h. (b) Experimental setup indicating the therapy and imaging transducers. The sound beam first goes through a water column before entering the liver. Microbubbles were injected and contrast-enhanced ultrasound images taken. (c) Imaging of microbubble extravasation possibly as a result of vessel perforation after ultrasound exposure. The peak negative pressure was 4 MPa with 1000 cycles. (d) Image of the treated area after 60 min of ultrasound exposure at 1.7 MPa, 100 cycles. Some mechanical tissue damage is observed.
Abstract
Localized drug delivery and uptake can benefit from the combined action of ultrasound and microbubbles at a specific site. Some of the possible mechanisms suggested are vessel poration and/or cell poration, but the exact acoustic parameters that trigger those phenomena remain unknown. Ex vivo machine perfusion of human-sized organs is a technique that provides an ideal environment for pre-clinical investigations with high physiologic relevance not possible with in vitro experiments. In this work, ex vivo machine-perfused pig livers were combined with an image-guided therapy system to investigate microvascular flow changes caused by the interaction of ultrasound-driven microbubbles with the vasculature. The effects of acoustic pressure (1.7-4 MPa peak negative pressures) and number of cycles (1000 or 20 cycles) were examined. Perfusion changes caused by the action of ultrasound on microbubbles in the microcirculation were qualitatively and quantitatively assessed with contrast-enhanced ultrasound and used as a metric of the extent of vessel perforation, thus, extravasation. Areas that were exposed to peak negative pressures above 1.7 MPa underwent a detectable and irreversible perfusion change. Complete devascularization of the area exposed to ultrasound was observed at much larger acoustic pressures (?4 MPa). Shorter acoustic pulses (20 cycles) produced markedly fewer perfusion changes than longer pulses (1000 cycles) under the same acoustic amplitude exposure.