Research Associate Professor
Email: drewfus@uw.edu
Phone: (206) 616-8023
Office: Foege N510C

Drew Sellers
We are inventing the future of medicine by developing powerful new peptides for drug delivery into the central nervous system, hydorgels that “sense” and “respond” to the tissue micro-environment, and stem cell reprogramming strategies to drive neurogenesis in the injured spinal cord. These technologies have the potential to revolutionize treatments for post-traumatic injury and neurodegenerative disease in the CNS such as ALS, Alzheimer’s Disease, and Brain or Spinal Cord Injury.
Cell reprogramming strategies
Neural Regeneration
“Smart” Biomaterial and bio-responsive hydrogels
Drug delivery
Stem Cell Biology and Technology
Tissue Engineering and nanomedecine
Despite possessing a resident pool of neural stem cells, the mammalian brain and spinal cord shows a limited ability to regenerate damaged tissue after traumatic injury. Instead, injury initiates a cascade of events that direct reactive gliosis to wall off an injury with a glial scar to mitigate damage and preserve function. My current research interests explore approaches to re-engineer the stem cell niche, to utilize gene-therapy and genome editing approaches to reprogram and engineer stem cells directly, and to enhance drug delivery into the central nervous system (CNS) to drive regenerative strategies that augment functional recovery in the diseased or traumatically injured CNS.
Ph.D., Molecular and Cell Biology, Oregon State University, 2001
B.S., Biology, The College of Idaho, 1994
Choi, J.L., Tan, J-K. J., Sellers, D.L., Wie, H., Horner, P.J., Pun, S.H. (2015). Guanidinylated block copolymers for gene transfer: A comparison of in vitro and in vivo gene transfer efficiency. Biomaterials, 54(C), 87–96. doi:10.1016/j.biomaterials.2015.03.008
Sellers, D.L., Kim, T.H., Mount, C., Pun, S.H., and Horner, P.J. (2014). Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion. Biomaterials, 35(31), 8895–8902. doi:10.1016/j.biomaterials.2014.06.051.
Chu, D.S.H. and Sellers, D.L., Bocek M.J., Fischedick A.E., Horner, P.J., and Pun, S.H. (2014). MMP9-sensitive polymers mediate environmentally-responsive bivalirudin release and thrombin inhibition. (2015). Biomater Sci 3(1), 41–45. doi:10.1039/C4BM00259H
Wei, H., Volpatti, L. R., Sellers, D. L., Maris, D. O., Andrews, I. W., Hemphill, A. S., et al. (2013). Dual Responsive, Stabilized Nanoparticles for Efficient In Vivo Plasmid Delivery. Angewandte Chemie International Edition, 52(20) 5377-5381
Powers, B. E., Sellers, D.L., Lovelett E. A., Cheung W., Aalami S. P., Zapertov N., Maris, D.O., Horner, P.J. (2013). Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Prod Natl Acad Sci 110(10):4075-4080
Sellers, D.L., Maris, D.O., Horner, P.J. (2009). Post-injury Niches Induce Temporal Shifts in Progenitor Fates to Direct Lesion Repair after Spinal Cord Injury. Journal of Neuroscience 29(20):6722-33
Petit, A., Sellers, D.L., Liebl, D.J., Tessier-Levigne, M., Kennedy, T.E., Horner, P.J. (2007) Adult spinal cord porgenitor cells are repelled by netrin-1 in the embryonic and injured adult spinal cord. Proc Natl Acad Sci 104(45):17837-4.
In the News
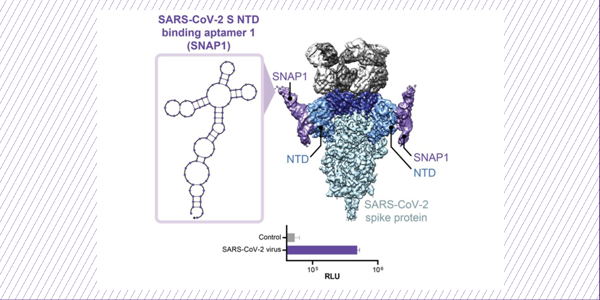
Discovery and Characterization of Spike N-Terminal Domain-Binding Aptamers for Rapid SARS-CoV-2 Detection
2022-01-06T15:37:50-08:00December 6th, 2021|
TAxI peptide shuttles protein cargo into spinal cord
2020-10-26T08:30:03-07:00February 17th, 2016|