UW Bioengineering Professor Dr. Valerie Daggett and research team members have designed a peptide structure that can stop harmful changes of proteins in the body that are linked to diseases such as Alzheimer’s, Parkinson’s, heart disease, Type 2 diabetes and Lou Gehrig’s disease. The researchers’ findings were published online this month in the journal eLife.
The team, which includes UW Bioengineering professor Dr. James Bryers, aimed to “catch and neutralize the toxic version of the proteins” associated with these illnesses, known as amyloid diseases, explains Dr. Daggett. These diseases are thought to be caused by the buildup of proteins that have transformed from a normal to an abnormal state. The structure of an amyloid protein is often just slightly different from a normal protein and can change to a toxic state quickly. And while many amyloid diseases present similar symptoms, identifying which exact protein or peptide is responsible usually does not occur until a patient dies and an autopsy is conducted. Thus, many patients, such as those with dementia, receive an Alzheimer’s diagnosis, and those with other diseases may not receive appropriate diagnosis or treatment.
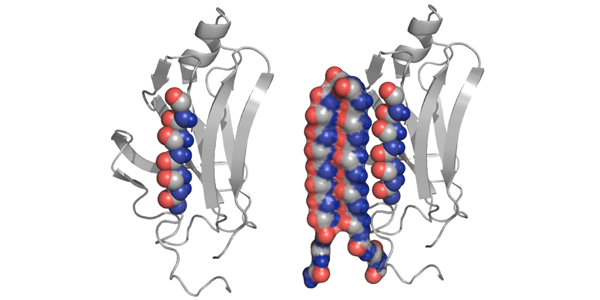
The researchers discovered through computer simulations that amyloid proteins undergo a toxic, intermediate stage. They built a protein structure, called “alpha sheet” that complements this toxic structure. The alpha sheet binds to, attacks and neutralizes the middle stage proteins undergo as they transition from normal to abnormal.
The team is currently working with UW’s Center for Commercialization (C4C) to patent their compounds and hope that their work leads to new ways to diagnose and treat amyloid diseases. Their concept could be refined further to bind only with the proteins associated with certain diseases, allowing a physician to identify exactly which disease a patient has.