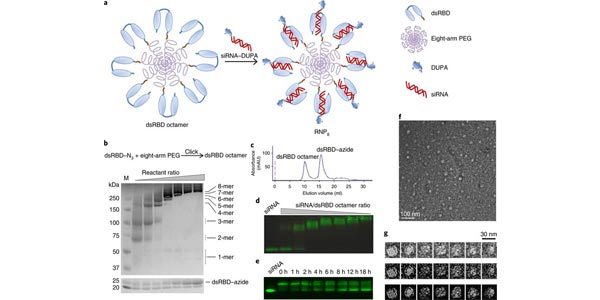
Image: Schematic and characterization of RNP8 and its intermediates. View full graphical abstract information.
A ribonucleoprotein octamer for targeted siRNA delivery
Wanyi Tai, Junwei Li, Eva Corey and Xiaohu Gao
Nature Biomedical Engineering. Volume 2, Pages 326–337 (2018)
Abstract
Hurdles in cell-specific delivery of small interfering RNA (siRNA) in vivo hinder the clinical translation of RNA interference (RNAi). A fundamental problem concerns conflicting requirements for the design of the delivery vehicles: cationic materials facilitate cargo condensation and endosomolysis, yet hinder in vivo targeting and colloidal stability. Here, we describe a self-assembled, compact (~30?nm) and biocompatible ribonucleoprotein-octamer nanoparticle that achieves endosomal destabilization and targeted delivery. The protein octamer consists of a poly(ethylene glycol) scaffold, a sterically masked endosomolytic peptide and a double-stranded RNA-binding domain, providing a discrete number of siRNA loading sites and a high siRNA payload (>30?wt%), and offering flexibility in both siRNA and targeting-ligand selection. We show that a ribonucleoprotein octamer against the polo-like kinase 1 gene and bearing a ligand that binds to prostate-specific membrane antigen leads to efficient gene silencing in prostate tumour cells in vitro and when intravenously injected in mouse models of prostate cancer. The octamer’s versatile nanocarrier design should offer opportunities for the clinical translation of therapies based on intracellularly acting biologics.