
Mike Averkiou and doctoral student Connor Krolak reviewing ultrasound images of tumor perfusion with microbubbles from a patient study. Photo by Dennis Wise.
The project will develop an approach that uses ultrasound and microbubbles to treat hepatocellular carcinoma (HCC) more successfully and with less toxicity and risks than current methods.
Mike Averkiou, associate professor of UW Bioengineering, received a $3 million R01 grant from the National Institutes of Health to treat hepatocellular carcinoma (HCC), the most common form of liver cancer, through ultrasound and microbubble-mediated cavitation.
HCC is the fifth most common type of cancer with an estimated 750,000 diagnosed cases per year, making it the third leading cause of cancer-related deaths worldwide. Because of the advanced stage of the disease at the time of diagnosis, less than 30-40% of HCC patients are eligible for potentially curative therapies including surgical removal, transplantation or ablation.
Most patients with HCC who are not candidates for surgical removal or ablation are treated with chemotherapeutic drugs that in sufficient concentrations are potent enough to kill cancer cells. However, these treatments result in limited improvements in patient survival rates because the abnormal tumor microenvironment makes it difficult for the drugs to reach the cancer cells and be effective.
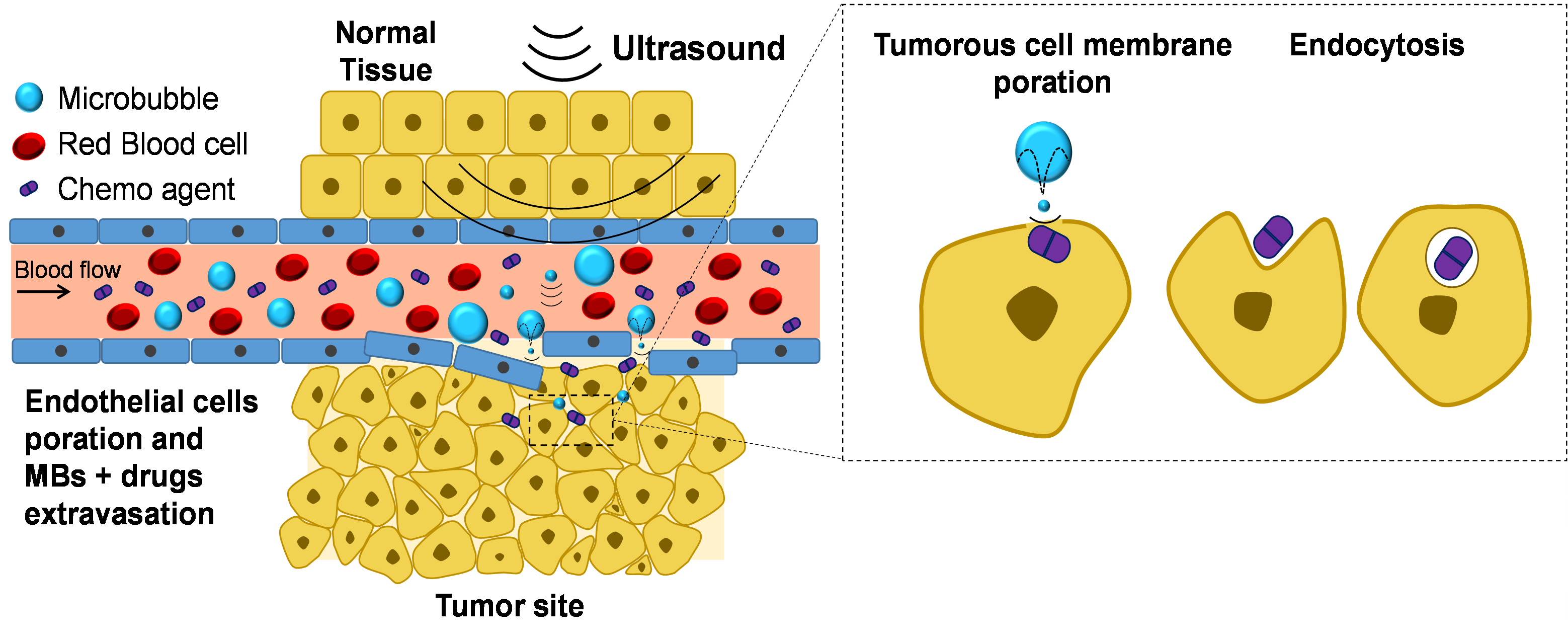
A schematic showing how cavitation treatments affect the tumor microenvironment. Courtesy of Mike Averkiou.
The grant will allow Averkiou and his team to develop a method that uses ultrasound and microbubbles to locate the tumor, sensitize it and make it more receptive to chemotherapeutic drugs. This approach may increase the therapeutic efficacy due to its precise targeting, which reduces systemic toxicity and other chemotherapy-related risks by requiring lower drug doses. Additionally, since the new method relies on sensitizing the tumor microenvironment, it will work with both existing and future drugs currently in development
The combined action of ultrasound and microbubbles on cells and blood vessels leads to changes in the tumor microenvironment that promote drug uptake.
Mike Averkiou
The power of microbubbles
Microbubbles are currently used clinically as ultrasound contrast agents and are approved by the Food and Drug Administration for adult and pediatric liver use. They dramatically enhance ultrasound images of the tumor vasculature. These tiny bubbles are the same size as red blood cells, which are much smaller than the diameter of a human hair. After they are delivered intravenously, the microbubbles circulate in the blood for about 15 minutes until they are eliminated by the lungs or the liver. Due to their size, they do not escape the vascular system and are thus called a “blood pool” agent. Microbubbles have many imaging applications including organ blood flow evaluation, tumor detection and characterization, and monitoring the remodeling of tumor vessels during the course of chemotherapy treatments.
Ultrasound cavitation treatment
Using microbubbles with ultrasound at a higher amplitude than the amplitude used in imaging creates unique microbubble oscillations which can enhance chemotherapy drug uptake in patients with drug resistant tumors. This procedure is called ultrasound cavitation treatment. Research has shown that, under certain conditions, this treatment can safely create pores in the tumor vessels and cells, allowing chemotherapy drugs to penetrate the tumor. “The combined action of ultrasound and microbubbles on cells and blood vessels leads to changes in the tumor microenvironment that promote drug uptake,” Averkiou said.
Ultrasound scanning of a liver tumor with microbubbles. Pictured above (l-r): Mike Averkiou, Shaun Bornemeier (Sonographer), patient, Manjiri Dighe (Radiologist). Photo by Dennis Wise.
Cavitation treatments may be accurately guided with diagnostic ultrasound imaging to precisely target the tumor. Changes to the tumor microenvironment can be confirmed with contrast-enhanced ultrasound and shear wave elastography, which measure blood flow changes and tissue stiffness in the tumor due to disease. A unique aspect of the research is that the same scanner and technology used for imaging the tumor vasculature and evaluating the tumor stiffness can also be used to create cavitation and treat tumors.
Similar past investigations with ultrasound and microbubbles primarily concentrated on targeting the tumor cells and did not explore targeting the tumor microenvironment for improved drug uptake. Previous studies also did not utilize a clinical ultrasound scanner. Instead, researchers attempted to create custom research systems that required regulatory approval for clinical use. Adapting a clinical scanner for ultrasound cavitation treatments will accelerate clinical research and clinical translation of the method. Seattle-based Philips Healthcare/Ultrasound is a collaborator that will implement the technology on a clinical scanner.
Mechanotherapeutic drugs
Collaborators of the Averkiou lab from the University of Cyprus have successfully repurposed several common drugs and evaluated their efficacy to act as mechanotherapeutic drugs on breast and pancreatic cancer tumors. The researchers observed therapeutic benefits from these drugs when combined with chemotherapy. Mechanotherapeutic drugs reduce tumor stiffness and pressure, resulting in decompression of tumor blood vessels which leads to enhanced delivery and efficacy of chemotherapies.
The combination of cavitation and mechanotherapeutics
Despite a small number of publications on drug enhancement with either mechanotherapeutics or ultrasound and microbubbles in animal tumor models, no work has combined the two techniques nor evaluated their synergistic effect on the liver tumor microenvironment during chemotherapy. “Image-guided ultrasound cavitation treatments combined with mechanotherapeutics and performed with a clinical ultrasound scanner presents an innovative approach for precision medicine for HCC,” Averkiou said. The procedure is expected to lead to better treatment outcomes and overall survival for HCC patients initially, and for other malignancies in the near future.
Mike Averkiou
Averkiou received his B.S., M.S. and Ph.D. in mechanical engineering/nonlinear acoustics from the University of Texas at Austin. His postdoc was in the Applied Physics Lab at the UW. From there, he worked for Phillips Ultrasound from 1996 to 2005. His time in industry led him to the University of Cyprus as assistant and then associate professor of mechanical engineering and biomedical engineering. Averkiou joined UW BioE in 2015 as associate professor where his lab has focused on developing imaging and therapy ultrasound technology to bring image-guided ultrasound-mediated drug delivery in clinical trials.
Read how Averkiou’s work using a combination of ultrasound and microbubbles helped diagnose and inform a successful treatment plan for a fellow UW bioengineering professor with cancer.
Article by Arden Clise

