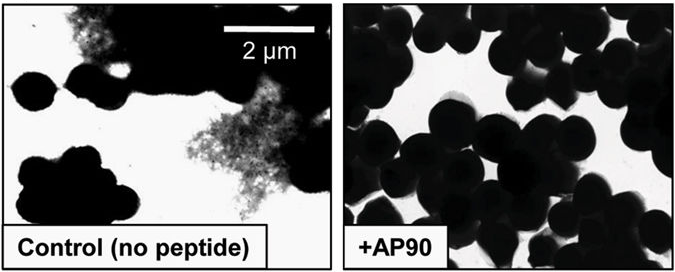
Image: S. aureus biofilm structures become less robust when grown in the presence of designed peptide inhibitors. S. aureus SH1000 WT biofilms grown in regular LB medium, PSM amyloid fibrils were visible as deposits in spaces between cells (left). Upon addition of the designed peptide AP90 (80?M), no extracellular fibril deposits were observed (right)
Abstract
Nosocomial infections affect hundreds of millions of patients worldwide each year, and ~60% of these infections are associated with biofilm formation on an implanted medical device. Biofilms are dense communities of microorganisms in which cells associate with surfaces and each other using a self-produced extracellular matrix composed of proteins, polysaccharides, and genetic material. Proteins in the extracellular matrix take on a variety of forms, but here we focus on functional amyloid structures. Amyloids have long been associated with protein misfolding and neurodegenerative diseases, but recent research has demonstrated that numerous bacterial species utilize the amyloid fold to fortify the biofilm matrix and resist disassembly. Consequently, these functional amyloids, in particular the soluble oligomeric intermediates formed during amyloidogenesis, represent targets to destabilize the extracellular matrix and interrupt biofilm formation. Our previous studies suggested that these amyloidogenic intermediates adopt a non-standard structure, termed “?-sheet”, as they aggregate into soluble oligomeric species. This led to the design of complementary ?-sheet peptides as anti-?-sheet inhibitors; these designs inhibit amyloidogenesis in three unrelated mammalian disease-associated systems through preferential binding of soluble oligomers. Here we show that these anti-?-sheet peptides inhibit amyloid formation in Staphylococcus aureus biofilms. Furthermore, they inhibit aggregation of pure, synthetic phenol soluble modulin ?1, a major component of Staphylococcus aureus functional amyloids. As it aggregates phenol soluble modulin ?1 adopts ?-helix then ?-sheet and finally forms ?-sheet fibrils. The binding of the designed peptide inhibitors coincides with the formation of ?-sheet.