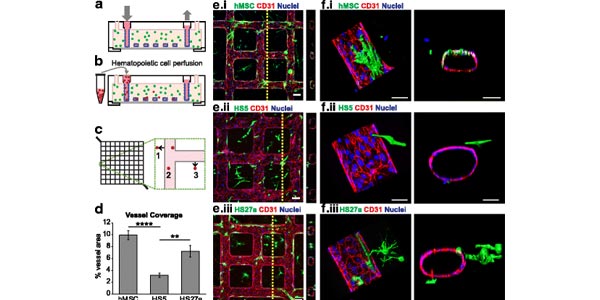
Image: Coculture of marrow fibroblasts with engineered vessels create perfusable marrow microenvironments. a Schematic of microvessel platform shows a perfusable network that is then b perfused with hematopoietic cells. c Perfused hematopoietic cells are identified as migrated (1, 3) or adhered (2) within a portion of the vessel network. d Quantification of percent vessel coverage by human mesenchymal stem cells (hMSCs) and marrow fibroblast types show that hMSCs and HS27a cells coat vessels more than HS5 fibroblasts. ****p < 0.0001, **p < 0.005. e Cocultured hMSCs and marrow fibroblasts interact with endothelial vessel walls. Scale bars = 100 ?m. f Three-dimensional reconstruction and orthogonal views of co-cultured microvessels show surface renderings of MSCs, HS5, and HS27a cells interacting with the endothelial wall. Scale bars = 50 ?m
Engineering a multicellular vascular niche to model hematopoietic cell trafficking
Surya S. Kotha, Brian J. Hayes, Kiet T. Phong, Meredith A. Redd, Karol Bomsztyk, Aravind Ramakrishnan, Beverly Torok-Storb and Ying Zheng
Stem Cell Research & Therapy. 2018, 9:77.
Abstract
Background
The marrow microenvironment and vasculature plays a critical role in regulating hematopoietic cell recruitment, residence, and maturation. Extensive in vitro and in vivo studies have aimed to understand the marrow cell types that contribute to hematopoiesis and the stem cell environment. Nonetheless, in vitro models are limited by a lack of complex multicellular interactions, and cellular interactions are not easily manipulated in vivo. Here, we develop an engineered human vascular marrow niche to examine the three-dimensional cell interactions that direct hematopoietic cell trafficking.
Methods
Using soft lithography and injection molding techniques, fully endothelialized vascular networks were fabricated in type I collagen matrix, and co-cultured under flow with embedded marrow fibroblast cells in the matrix. Marrow fibroblast (mesenchymal stem cells (MSCs), HS27a, or HS5) interactions with the endothelium were imaged via confocal microscopy and altered endothelial gene expression was analyzed with RT-PCR. Monocytes, hematopoietic progenitor cells, and leukemic cells were perfused through the network and their adhesion and migration was evaluated.
Results
HS27a cells and MSCs interact directly with the vessel wall more than HS5 cells, which are not seen to make contact with the endothelial cells. In both HS27a and HS5 co-cultures, endothelial expression of junctional markers was reduced. HS27a co-cultures promote perfused monocytes to adhere and migrate within the vessel network. Hematopoietic progenitors rely on monocyte-fibroblast crosstalk to facilitate preferential recruitment within HS27a co-cultured vessels. In contrast, leukemic cells sense fibroblast differences and are recruited preferentially to HS5 and HS27a co-cultures, but monocytes are able to block this sensitivity.
Conclusions
We demonstrate the use of a microvascular platform that incorporates a tunable, multicellular composition to examine differences in hematopoietic cell trafficking. Differential recruitment of hematopoietic cell types to distinct fibroblast microenvironments highlights the complexity of cell-cell interactions within the marrow. This system allows for step-wise incorporation of cellular components to reveal the dynamic spatial and temporal interactions between endothelial cells, marrow-derived fibroblasts, and hematopoietic cells that comprise the marrow vascular niche. Furthermore, this platform has potential for use in testing therapeutics and personalized medicine in both normal and disease contexts.