Harnessing Sphingosine-1-Phosphate Signaling and Nanotopographical Cues
Jonathan H. Tsui, Kajohnkiart Janebodin, Nicholas Ieronimakis, David M. P. Yama, Hee Seok Yang, Rakchanok Chavanachat, Aislinn L. Hays, Haeshin Lee, Morayma Reyes, and Deok-Ho Kim. ACS Nano 2017 11 (12), 11954-11968.
Abstract
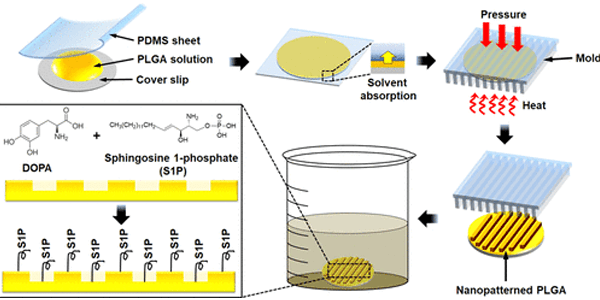
Despite possessing substantial regenerative capacity, skeletal muscle can suffer from loss of function due to catastrophic traumatic injury or degenerative disease. In such cases, engineered tissue grafts hold the potential to restore function and improve patient quality of life. Requirements for successful integration of engineered tissue grafts with the host musculature include cell alignment that mimics host tissue architecture and directional functionality, as well as vascularization to ensure tissue survival. Here, we have developed biomimetic nanopatterned poly(lactic-co-glycolic acid) substrates conjugated with sphingosine-1-phosphate (S1P), a potent angiogenic and myogenic factor, to enhance myoblast and endothelial maturation. Primary muscle cells cultured on these functionalized S1P nanopatterned substrates developed a highly aligned and elongated morphology and exhibited higher expression levels of myosin heavy chain, in addition to genes characteristic of mature skeletal muscle. We also found that S1P enhanced angiogenic potential in these cultures, as evidenced by elevated expression of endothelial-related genes. Computational analyses of live-cell videos showed a significantly improved functionality of tissues cultured on S1P-functionalized nanopatterns as indicated by greater myotube contraction displacements and velocities. In summary, our study demonstrates that biomimetic nanotopography and S1P can be combined to synergistically regulate the maturation and vascularization of engineered skeletal muscles.