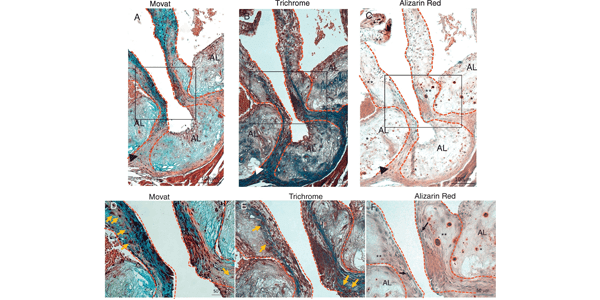
Image: Movat (A and D), Trichrome (B and E), and Alizarin Red (C and F) staining of aortic sinus adjacent sections from DB fed mice. D, E and F are higher magnification of insets in A, B and C respectively. Dotted lines contour the valve leaflets and hinge areas. (A-C) Chondrocyte-like cells in hinge area co-localize with dense collagen and calcium deposits (arrow-heads). Extensive calcification in the leaflets (**, C and F) is associated with collagen and proteoglycan rich areas. Extensive calcification is also present in the atherosclerotic lesion (AL) (*, C and F) associated with collagen and proteoglycan rich areas. Chondrocyte-like cells are also present in the leaflets and hinge areas (orange arrows, D and E). Melanocytes (black arrows, F) are present in the leaflets. AL=atherosclerotic lesion areas, **=leaflet calcification, *=atherosclerotic lesion calcification, arrow-head=calcification and chondrocyte cells in the hinge areas, orange arrows=chondrocyte-like cells.
Marta Scatena, Melissa F. Jackson, Mei Y. Speer, Elizabeth M. Leaf, Mary C. Wallingford, Cecilia M. Giachelli. Cardiovascular Pathology. Volume 34, May-June 2018, Pages 28-37.
Abstract
Objective
Calcific aortic valve disease (CAVD) is a major cause of aortic stenosis (AS) and cardiac insufficiency. Patients with type II diabetes mellitus (T2DM) are at heightened risk for CAVD, and their valves have greater calcification than nondiabetic valves. No drugs to prevent or treat CAVD exist, and animal models that might help identify therapeutic targets are sorely lacking. To develop an animal model mimicking the structural and functional features of CAVD in people with T2DM, we tested a diabetogenic, procalcific diet and its effect on the incidence and severity of CAVD and AS in the, LDLr-/-ApoB100/100 mouse model.
Results
LDLr-/-ApoB100/100 mice fed a customized diabetogenic, procalcific diet (DB diet) developed hyperglycemia, hyperlipidemia, increased atherosclerosis, and obesity when compared with normal chow fed LDLr-/-ApoB100/100 mice, indicating the development of T2DM and metabolic syndrome. Transthoracic echocardiography revealed that LDLr-/-ApoB100/100 mice fed the DB diet had 77% incidence of hemodynamically significant AS, and developed thickened aortic valve leaflets and calcification in both valve leaflets and hinge regions. In comparison, normal chow (NC) fed LDLr-/-ApoB100/100 mice had 38% incidence of AS, thinner valve leaflets and very little valve and hinge calcification. Further, the DB diet fed mice with AS showed significantly impaired cardiac function as determined by reduced ejection fraction and fractional shortening. In vitro mineralization experiments demonstrated that elevated glucose in culture medium enhanced valve interstitial cell (VIC) matrix calcium deposition.
Conclusions
By manipulating the diet we developed a new model of CAVD in T2DM, hyperlipidemic LDLr-/-ApoB100/100 that shows several important functional, and structural features similar to CAVD found in people with T2DM and atherosclerosis including AS, cardiac dysfunction, and inflamed and calcified thickened valve cusps. Importantly, the high AS incidence of this diabetic model may be useful for mechanistic and translational studies aimed at development of novel treatments for CAVD.