Yen-Wen Liu, Billy Chen, Xiulan Yang, James A Fugate, Faith A Kalucki, Akiko Futakuchi-Tsuchida, Larry Couture, Keith W Vogel, Clifford A Astley, Audrey Baldessari, Jason Ogle, Creighton W Don, Zachary L Steinberg, Stephen P Seslar, Stephanie A Tuck, Hiroshi Tsuchida, Anna V Naumova, Sarah K Dupras, Milly S Lyu, James Lee, Dale W Hailey, Hans Reinecke, Lil Pabon, Benjamin H Fryer, W Robb MacLellan, R Scott Thies anbd Charles E Murry
Nature Biotechnology 36, 597-605 (2018).
Abstract
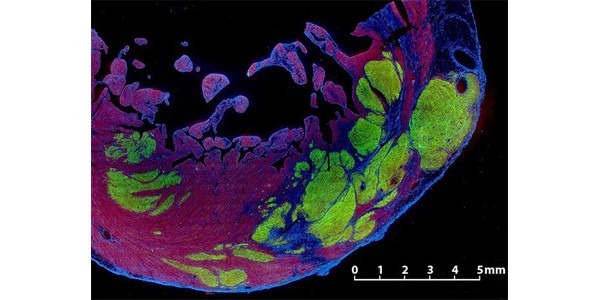
Pluripotent stem cell–derived cardiomyocyte grafts can remuscularize substantial amounts of infarcted myocardium and beat in synchrony with the heart, but in some settings cause ventricular arrhythmias. It is unknown whether human cardiomyocytes can restore cardiac function in a physiologically relevant large animal model. Here we show that transplantation of ?750 million cryopreserved human embryonic stem cell–derived cardiomyocytes (hESC-CMs) enhances cardiac function in macaque monkeys with large myocardial infarctions. One month after hESC-CM transplantation, global left ventricular ejection fraction improved 10.6 ± 0.9% vs. 2.5 ± 0.8% in controls, and by 3 months there was an additional 12.4% improvement in treated vs. a 3.5% decline in controls. Grafts averaged 11.6% of infarct size, formed electromechanical junctions with the host heart, and by 3 months contained ?99% ventricular myocytes. A subset of animals experienced graft-associated ventricular arrhythmias, shown by electrical mapping to originate from a point-source acting as an ectopic pacemaker. Our data demonstrate that remuscularization of the infarcted macaque heart with human myocardium provides durable improvement in left ventricular function.