New non-invasive method may help detect heart attacks, monitor healing after bypass procedure and improve image-guided plastic surgery.
Collagen, the most abundant protein in the body, provides the structure and mechanical support for skin, bones, muscles and more. The way collagen is organized plays an important role in many processes, from wound healing and scar formation to heart attacks and cancer.
Ricky Wang, UW professor of bioengineering and of ophthalmology
Imaging tools that help doctors see how collagen fibers are organized are critical for an accurate diagnosis of disease and the best outcomes in surgery, especially in dermatology. However, current medical imaging technologies have a range of limitations, from requiring a biopsy that is invasive to having such low resolution that it’s impossible to interpret the microscopic information.
Now, a team of University of Washington bioengineers has developed a new non-invasive method for imaging collagen structures deep within tissue in 3D. In proof-of-principle experiments, they tested their system in rodent hearts and living human skin.
The team, led by Ruikang (Ricky) Wang, UW professor of bioengineering and of ophthalmology, published these results Nov. 24 in Nature’s Light: Science & Applications.
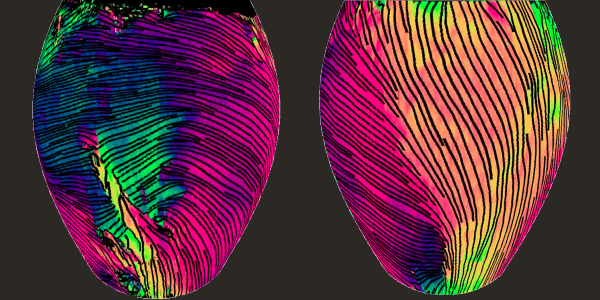
In its experiments, the team tested its new method first in hearts from healthy rats and then in a rat heart that had undergone a heart attack. They showed their method can map and reconstruct the heart myofiber orientation of a whole mouse heart, including the typical helical-type structure. In the oxygen-starved heart tissue, although the surface collagen appeared normal, deep within the cardiac wall their imaging revealed random and disjointed patterns, a hallmark of damage from a heart attack.
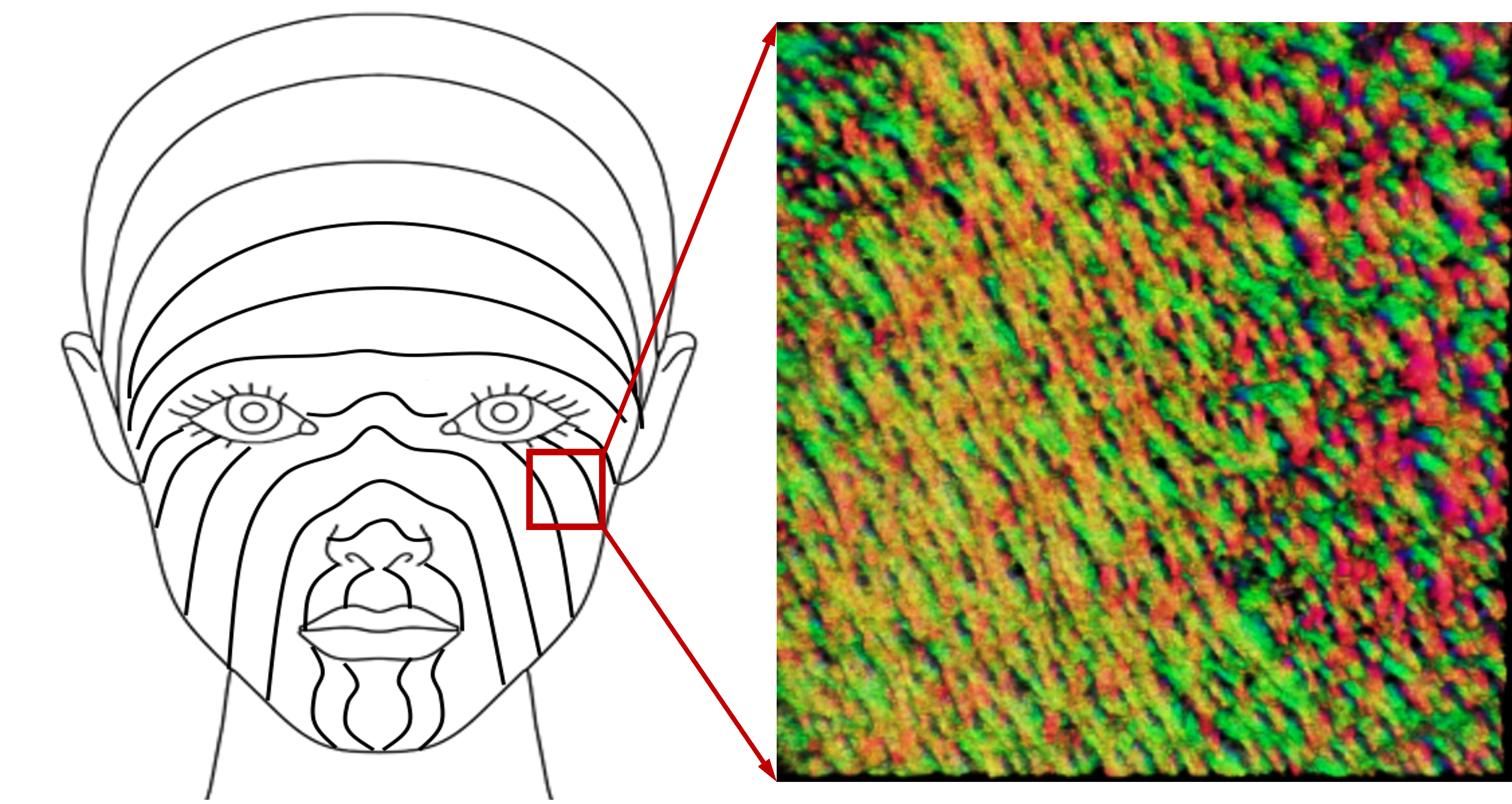
The new technique is able to clearly identify collagen organization and skin tension lines in the face. Image-guided surgery could help reconstructive surgeons achieve the best results.
They also tested the technique on a healthy volunteer’s skin around the eyes and cheek, revealing the underlying collagen structure responsible for facial tension lines in human skin. Knowing how collagen fibers naturally lie under the skin can help surgeons plan and place patient-specific, guided incisions for the best outcomes and least scarring in reconstructive surgery and cosmetology.
“These findings are extremely valuable for a number of clinical applications, such as early detection of acute heart attack, monitoring of the graft remodeling following bypass surgery after a heart attack, evaluation of scar formation and imaging-guided plastic surgery,” said Dr. Wang, who is also the WRF/David and Nancy Auth Innovator of Bioengineering and Jules and Doris Stein Research to Prevent Blindness Professor of Ophthalmology.
“These findings are extremely valuable for a number of clinical applications, such as early detection of acute heart attack, monitoring of the graft remodeling following bypass surgery after a heart attack, evaluation of scar formation and imaging-guided plastic surgery.” – Dr. Ricky Wang
Simplicity of design
The basic protein nature of collagen causes light to split and travel at different speeds or along different paths, causing unwanted artifacts in imaging. To compensate for this, 3D imaging technology called optical coherence tomography (OCT) is augmented with a polarization sensitive extension (PSOCT). However, traditional PSOCT isn’t good at properly reconstructing collagen maps deep within tissue.
The UW researchers applied a discrete differential geometry-based polarization tracing method to PSOCT to enable superior contrast and resolve both micro- and macroscopic structural features.
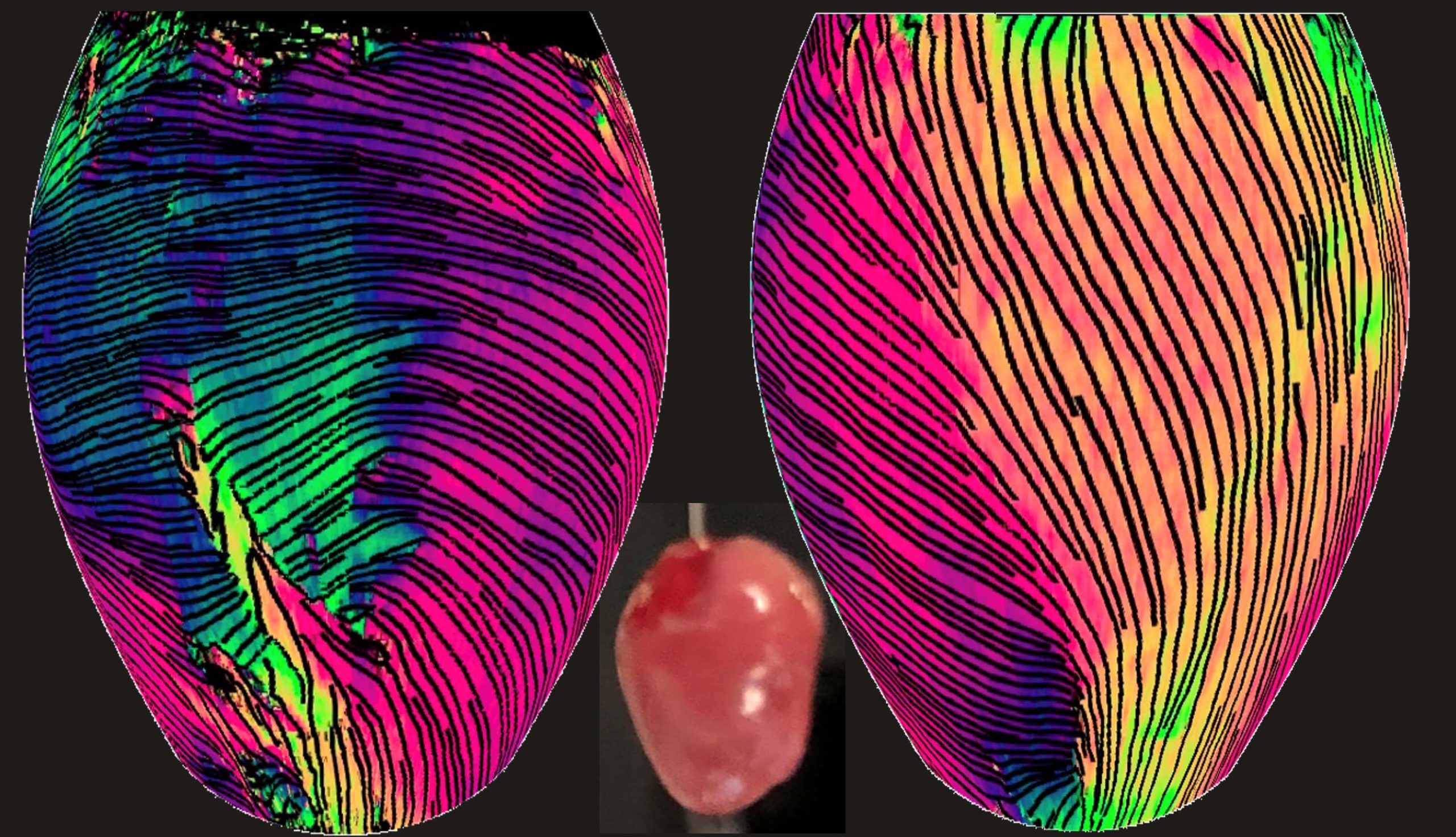
Here the imaging method reveals the orientation of myocardial fiber within the superficial ventricular wall of a whole mouse heart. The reconstructed 3D views above show the front of the heart (left) and the right-side view of the left ventricle (right). At center is a photo of a mouse heart.
Another benefit of the technique is that it uses a single input polarization state rather than many, simplifying the image system setup and reducing errors caused by motion. It’s also compatible with most PSOCT system designs that use a single input polarization state, providing a practical path for clinical translation.
“The significant simplification of the PSOCT imaging system facilitates the clinical translation of this imaging technique, which would provide a new matrix of diagnostic information,” Dr. Wang said.
The lead author on the paper is Peijun Tang, a bioengineering Ph.D. student in the Wang lab. Other co-authors are Mitchell A. Kirby, Nhan Le, Yuandong Li, Nicole Zeinstra, G. Nina Lu, assistant professor of otolaryngology, Charles Murry, joint professor of laboratory medicine and pathology, bioengineering, and cardiology, and Ying Zheng, associate professor of bioengineering.
This research was supported by grants from the National Institutes of Health (R01EY028753, R01HL141570, R01AR077560), the Washington Research Foundation, the Research to Prevent Blindness, Inc., and an NSF graduate fellowship (No. DGE-1256082).