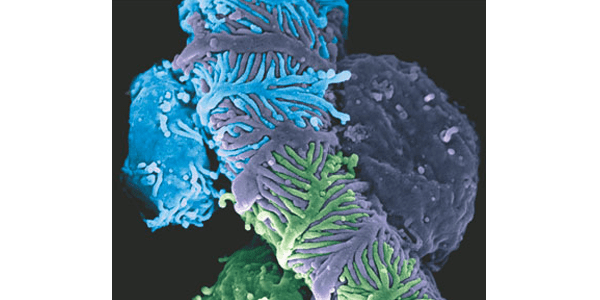
Graphic: Scanning electron micrograph of murine podocytes enwrapping a glomerular capillary with their foot processes. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Nephrology, Vol 9 No 11, copyright 2013.
At the age of five, second-year UW Bioengineering Ph.D. student Gary Liu was diagnosed with a chronic condition called minimal change kidney disease. His experience with the disease inspired him to study bioengineering. “Our ability to treat certain diseases such as mine are limited,” Gary explains. “More research and innovation is needed for an effective cure.”

UW Bioengineering Ph.D. student Gary Liu
Now, as an NSF Graduate Research Fellow in UW Bioengineering Professor Suzie Pun’s lab and collaborator with UW Nephrology chair and clinician Stuart Shankland, Gary investigates novel applications of biomaterials and drug delivery to treat kidney disease. Gary decided to attend UW due to the strong, early support he received for his research vision. His research mentors’ enthusiasm for his project is helping Gary fulfill a personal goal – improving his health – and also help other people suffering from kidney disease. “I can finally begin to address my own disease, for myself and others.”
With bioengineering, Gary takes his health care into his “own hands”
Minimal change kidney disease affects the glomeruli, a network of tiny vessels within the kidneys that filter waste products from blood and produce urine. In normally functioning kidneys, specialized cells within the glomeruli called podocytes interweave together, forming a structure that maintains the filtration function of the kidney. This filtration process keeps essential materials, such as protein within the blood, and passes waste products into urine. The proteins that stay behind continue to circulate in blood, helping keep fluid flowing through the body.
Minimal change kidney disease damages podocytes’ ability to network together, causing the filtration system of the kidney to malfunction. As the kidneys cannot filter urine correctly, protein leaks from the body in urine. Without protein moving it along, fluid starts to over-accumulate within the body, resulting in uncomfortable swelling and weight gain. Because the level of protein in blood is low, the body resorts to using up the protein stored in the muscles, which can result in fatigue and muscle wasting. Patients must to watch their diet and avoid excess sodium and fluid intake to alleviate swelling and reduce the risk of developing high blood pressure. In rare cases, the disease can progress to advanced kidney disease, damage and failure.
In most cases, minimal change kidney disease is treated successfully and resolves with few lasting complications. However, drugs used to treat the disease and other conditions affecting the kidneys, including steroids and immunosuppressants, can cause serious long-term side effects. Because such drugs lack the ability to target diseased tissue and instead act broadly on the body, their use can result in increased risk of infections, osteoporosis, glaucoma, cataracts and diabetes. Their use can lead to other, more serious side effects including kidney toxicity, obesity and growth retardation.
Gary’s doctors told him that he too would recover quickly. They often said, “You’ll get over this when you grow older,” he explains. “This is a disease that is supposed to resolve itself.” However, this wasn’t the case for Gary. When he entered high school, he still had the disease. At that point, he decided to take a proactive approach to improving his own health. “I started taking more initiative for taking my own health care into my own hands.” To Gary, studying bioengineering was the perfect way to achieve this goal.
As an undergraduate at the University of Texas at Austin, Gary explored the possibilities of drug delivery for treating kidney disease. “I thought that was really attractive, especially for kidney disease. We can target drugs to diseased tissues specifically; we can mitigate a lot of the side effects and improve the efficacy,” he explains. “Those were advantages that I saw that could really impact the nephrology field.”
At UW Bioengineering, Gary finds low barriers to clinical collaboration
Gary decided to attend graduate school to continue developing drug delivery strategies for treating kidney disease. To him, UW Bioengineering stood apart from other graduate programs due to its strong drug delivery research. “My undergraduate adviser had suggested UW based on my interest in drug delivery,” he says.
In 2012, Gary visited BioE’s booth at the BMES Annual Meeting in Atlanta to learn more about the department and UW. After talking to BioE’s graduate students, he was impressed with the department’s low barriers to collaboration with partners in UW’s College of Engineering and School of Medicine. He also liked the how close BioE is to UW’s medical school – UT Austin did not have its own medical school, which made clinical collaborations difficult. At UW, he explains, “I thought it was really cool that the med school was right across the street.”
When Gary applied to UW Bioengineering’s Ph.D. program, he was struck by the early strong support he received for his research proposal. In his application, he wrote about how his personal experience with kidney disease motivated him to pursue drug delivery research. He was surprised when Dr. Pun, a noted innovator in the field of drug delivery, contacted him to express her enthusiasm for his project. When Gary interviewed at UW, Dr. Pun offered to introduce him to Dr. Shankland, the UW nephrology chair and clinician, to discuss a potential research collaboration.
Gary received offers from several other graduate schools but ultimately decided on UW primarily because of Dr. Pun’s eager response to his research proposal. Gary was also impressed by the ease with which Dr. Pun was able to connect him with Dr. Shankland. “I thought that was really humbling, and really indicative of how UW treats students more like colleagues than students,” he says. “I thought that was really outstanding.”
When he arrived at UW, he found that Dr. Pun’s early initiative enabled him to start his project right away. “I’m still very awed by how quickly the collaboration came together,” Gary explains.
Future directions: “I think we can really change how kidney disease is managed”
Before Gary came to UW, Dr. Pun’s lab focused primarily on drug delivery for cancer and neurological disease. However, he found that the Pun lab’s expertise in developing biomaterials, as well as UW nephrology’s knowledge of disease biology, clinical needs and patient experiences, suited his research direction well. “The greatest benefit is that we have a lot of complementary knowledge that the other field may not necessarily have,” he says. “By talking to each other, by discussing what cells are the most attractive to deliver drugs to, we can formulate a strategy to design a material that can overcome the obstacles of delivery and get to the targeted cell.”
Gary is currently developing materials for kidney-targeted drug delivery by using peptide screening and polymer synthesis techniques that he has learned in the Pun lab, combined with kidney biology and mouse models from the Shankland lab. In the near future, he hopes to identify a peptide that can target and bind to podocytes, enabling direct drug delivery. “We believe that by directly delivering drugs to podocytes, we can deliver drugs directly to the site of disease and avoid a lot of side effects,” he says.
After completing his Ph.D., Gary plans to become a professor and define new directions for kidney disease treatment. He envisions starting a research program dedicated to translating bioengineering tools and technologies to clinical applications for treating kidney disease. “We know a lot about what causes kidney disease, but there hasn’t been a lot of translation of that knowledge in the engineering realm,” he says. “If we can take the technologies and platforms we develop in bioengineering and apply them to tackling kidney disease, then I think we can really change how kidney disease is managed.”