The world does not yet have a Star Trek tricorder. But UW bioengineers are developing devices and technology that may be powerful precursors to Dr. McCoy’s handy 23rd century diagnostic device, and may make improving health faster and easier than ever before. Researchers are answering the call for accessible, rapid testing tools, which can speed the time until treatment starts, helping prevent deaths, outbreaks and disability.
Hidden in blood, saliva and other body fluids lies a wealth of information about your health, from nutrition to whether you have the flu or HIV. Nowadays, to get at these nuggets of data, typically a sample ships off to a lab for expensive analysis by many machines and technicians. And then you – and your doctor – wait.
In poor countries with scant resources, the wait is longer. In Africa and Asia, where about 60 percent of the population lives in rural areas, some patients must travel on foot for miles to the nearest care provider. Often, the tests are just too expensive. But UW bioengineers are working to change that.
Rapid nutrition test
Health professionals know that when levels of five key micronutrients – folic acid, iron, iodine, vitamin A and zinc – fall too low, the risk of dying and disability from malnutrition goes up. Catching and treating deficiencies early, within the first 1,000 days, gives mothers and their developing children the best chance at health.
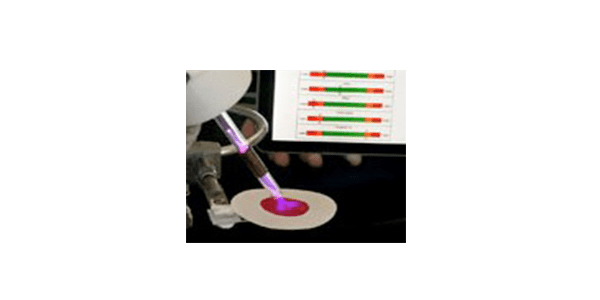
With this in mind, Buddy Ratner, professor of bioengineering and the Michael L. & Myrna Darland Endowed Chair in Technology Commercialization, set out to develop a device that screens for nutritional shortages and provides almost immediate test results. Aided by two rounds of funding from the Bill and Melinda Gates Foundation, he and his team coupled a plasma pencil with a mass spectrometer, and applied their expertise in multivariate data analysis to create a prototype wand that detects the barest trace of chemicals.
The hand-held plasma pencil atmospheric mass spectrometer, known as PPAMS, works by aiming the low-temperature glowing end of the pencil at a drop of blood or other sample. The charged gas plasma breaks ions free from the sample surface, and the mass spectrometer detects their weight and charge. The tool instantly analyzes the data, revealing which chemicals are present and at what level, and displays the results on a cell phone or tablet computer.
The prototype device can tease out a key handful of micronutrients from blood, which contains more than 4,000 chemical components. It currently measures iron, iodine and vitamin A levels in less than a minute. The goal, Ratner says, is to also measure folic acid and zinc and reveal all five results at once, showing a simple yes or no readout on a one-button device that almost anyone can use. This would let care providers treat a patient with the supplements they need on the spot.
Company formed
Ratner sees a number of other potential uses for the device. It can check cholesterol or most any other compound in blood and, he says, it could be used to find contaminants in food and water supplies, detect lead and bisphenol A in toys, screen for melanoma at the dermatologist’s office and even catch pharmaceutical drug counterfeiting.
In November 2013, Ratner, along with Jeanette Stein, project coordinator for the Gates-funded team and senior research scientist in bioengineering, and their colleagues formed a spinoff company, called ionReveal, to develop their PPAMS device for market. Ratner estimates the product will be finished in one to two years.
“We in Bioengineering are really motivated by this notion of impacting healthcare and addressing unmet needs,” Ratner says. “Our vision is that our plasma pencil will empower people to understand the complex chemistry in the world around them.”
Paper to the people
The plasma pencil is not the only testing tool that could bring rapid test results into the clinic, or even your home. A recent technology transforms ordinary paper into a versatile testing platform that could reduce health care costs in both the developed and developing world. Current specialized paper-based medical tests can test for pregnancy, diabetes and HIV, but they are expensive and limited in scope. Daniel Ratner, associate professor of bioengineering, and his team used common office paper, cheap solvent and a Ziploc bag to develop a simple system that they hope can be used for any type of medical test.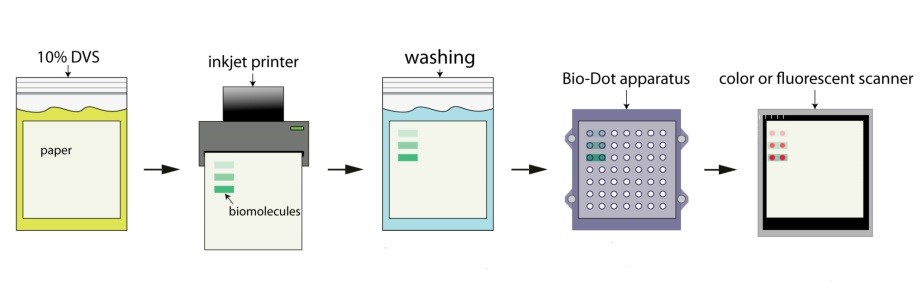
The team diluted divinyl sulfone, an industrial solvent used for decades as an adhesive, with water. They poured it into a Ziploc bag, added the stack of office paper, shook it for a couple of hours, and then rinsed and dried the paper. The result: paper that feels smooth to the touch but acts as sticky tape to chemicals. It grabs and holds proteins, nucleic acids and carbohydrates as well as sugars and small-molecule drugs.
To prove the concept, Daniel Ratner and his team modified an off-the-shelf ink-jet printer, replacing the ink in the cartridge with a biomolecule solution. Instead of printing ink, it “printed” a type of sugar on the treated paper in an invisible pattern. Exposing the paper to florescent ricin, a poison that bonds to the sugar, revealed the invisible pattern, showing that both the sugar and the poison were present.
Daniel Ratner and his team hope that researchers will take this flexible technology and apply it to their diverse needs. “We want to make a system that, regardless of the application, enables researchers,” Daniel Ratner said. “What are all the questions that people want to ask, and can we fashion a single versatile and universal substrate to support all these applications?”
Their next design challenge is refining and optimizing the device to make it behave in a predictable way with a variety of substances, such as blood, saliva and urine. “I’m interested in the interface of the paper with biology,” says Daniel Ratner. “Biology is fantastically complex, and it tends not to play well with contrived systems.”
Paper-based technologies
The Microfluidics 2.0 initiative, led by Paul Yager, professor of bioengineering, and Barry Lutz, research assistant professor of bioengineering, is developing a range 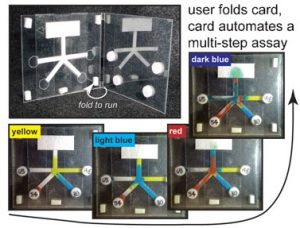 of new tests, including one that uses paper technology to print patterns of switchable networks.
of new tests, including one that uses paper technology to print patterns of switchable networks.
Yager and Lutz, along with Elain Fu, now assistant professor at Oregon State University and affiliate research assistant professor of bioengineering, teamed up nearly a decade ago to develop a new class of low cost, point-of-care medical test kits using microfluidics, which involves the design and control of tiny fluid channels and valves, like miniature plumbing networks. In 2008, researchers simplified microfluidic devices by building them using paper. Since then, the UW team has developed many new paper-based switch and valve systems that can control timing, re-creating the same complex reactions normally done by lab technicians or expensive lab equipment. In one example, a dissolvable sugar gate can delay reactions from minutes up to nearly an hour.
Tests on Fast Track
The team is partnering with other groups at UW, Seattle Children’s, PATH, GE Global Research and Epoch Biosciences to create fully disposable and very inexpensive test kits. They are on a fast track to be commercialized as lab-quality tests for use in the home, a doctor’s office, in the military or in the developing world. The tests aim to diagnose bacteria such as MRSA, viruses such as the ones that cause AIDS and the flu, and types of pathogens that cause malaria and other infectious diseases.
Some untrained users could perform the test at home using a sample such as a nasal swab or blood. After applying the sample to the paper, simply folding the card or sliding a plastic cover launches the process. Users can take a photo of the results and send them to a doctor via cell phone. The next step will be follow-up. “We want this to lead to interaction with the doctor to start treatment or recommend the next actions to the patient,” Lutz says. That may be visiting a doctor, doing a follow-up test, going to a drug store to pick up a prescription the doctor ordered, or getting plenty of rest and fluids, he suggests. The researchers note that cell phones are increasingly present in the developing world, where they can act as a vital link to health care systems.
The team has received nearly $6 million from NIH and over $13 million from the U.S. Department of Defense so far to develop prototypes for use in low-resource settings.
Faster, easier HIV screening
Lutz is also part of a team that’s working to develop a faster, easier test to screen for HIV drug resistance, so that those who have the drug-resistant form of HIV can begin the right drug treatment right away, boosting survival and reducing the odds of transmitting the disease to others.
Dr. Lisa Frenkel, a professor of pediatric infectious diseases and laboratory medicine at Seattle Children’s, has long worked to improve ways to detect and treat HIV and prevent mothers from transmitting the disease to their babies. To screen for HIV in places like the United States, doctors order a standard lab test that sequences the virus to determine its type, and then they prescribe the matching drugs. Costing up to $600, the test is out of reach for the developing world. Right now, patients in poor countries get standard antiretroviral drugs, even if the strain of HIV is resistant to them.
So Frenkel created a cheaper alternative to high-end sequencing screening tests. Moderately equipped labs can accommodate it, but since it requires skilled technicians, special lab instruments and about nine hours wait time, it’s still too complex for those with fewer resources. Now Lutz and James Lai, research assistant professor of bioengineering, are teaming with Frenkel to push her test further, to make it even faster and easier to use in places where well-equipped labs don’t exist.
Streamlining lab steps
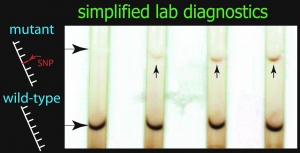
The team is re-designing the test to analyze a blood sample using a paper test strip. They are working to streamline the process for preparing the sample and concentrating the HIV target to make the test more sensitive. They’ve determined that the new test will be done with basic equipment, at one constant temperature. The test will replace dozens of complex procedures with a handful of simple steps that someone with little training can do. Processing time will drop from nine hours to about one hour, making it possible for patients to wait for the results. After exposing the test strip to chemicals that bind to certain forms of HIV, visible spots will appear on the paper, revealing whether the patient has a “wild-type” HIV strain or the drug-resistant form.
UW’s W.H. Coulter Foundation Translational Research Partnership program and Global WACh – the UW’s Global Center for Integrated Health of Women, Adolescents and Children – teamed up to fund the initial effort. Seattle Children’s Research also provided funding, and Frenkel heads up the team’s new five-year grant from the National Institutes of Health. The $2.3 million grant also adds Elitech/Epoch Biosciences as a collaborator. “Things are moving extraordinarily fast,” Lutz says. “Within a couple of years, we would like to have simplified lab versions and pilot a test in low-resource settings.”
Perhaps one day researchers will achieve the versatility of the Star Trek tricorder. Until then, the world may benefit from the technologies being developed today.
Article by Lia Unrau