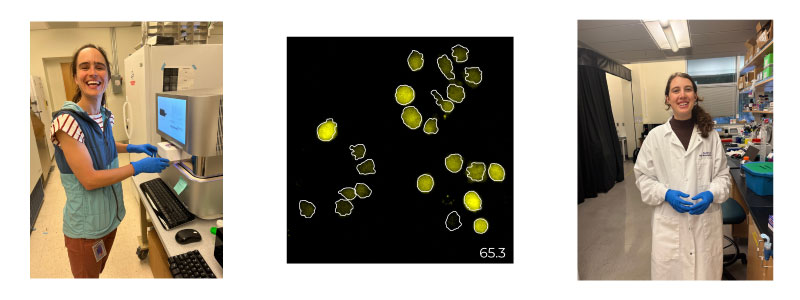
Co-leading authors Kathleen Abadie (left) Elisa Clark (right) and an image of differentiating T cells expressing a YFP reporter for the transcription factor TCF1, with the cell segmentation outlines in white (middle).
The immune system can remember an encounter with a pathogen, and quickly eliminate the same pathogen upon a later encounter. This immune memory underlies the remarkable protection that vaccines provide against dangerous diseases.
It has been known for decades that immune memory arises from the generation of memory lymphocytes – cells that can survive for an extended time after an infection clears and respond rapidly upon subsequent challenge. However, despite much study, scientists remained puzzled and widely debated how and when these memory cells form over the course of infection.
Kathleen Abadie and Elisa Clark, National Science Foundation graduate fellows in the Kueh Lab, focused on cytotoxic T cells, the immune cells responsible for directly eliminating infected cells. Using live-cell imaging, they observed memory formation in real-time in entire lineages of living cytotoxic T cells. These approaches allowed the researchers to pinpoint exactly when memory decisions are made, and how they are controlled at the molecular level. Their findings were published Jan. 31 in the journal Immunity from Cell Press.
These findings will help us engineer better disease-fighting T cells, that can persist longer in the body to mediate disease clearance. Hao Kueh, UW bioengineering associate professor and senior author on the study.
Their experiments revealed an unexpected degree of flexibility in memory decision making. The researchers identified distinct phases during the immune response where memory T cells are generated. After pathogen encounter, T cells decide early whether to form memory or become an effector cell, which has potent cell-killing abilities but is short lived. Interestingly, after pathogen clearance, effector cells can make a late decision to rejoin the memory pool.
The authors further uncovered the molecular switch that underlies flexible memory formation. They found this switch acts on the key memory regulatory gene Tcf7 and can turn off Tcf7 early in response to stimulatory signals due to infection. However, importantly, it was discovered this switch is reversible when the signals are gone which enables cells that have committed to the effector trajectory to reverse their course.
The researchers explored the reasons why T cells show such flexibility in their memory decisions. People can better adapt to uncertain circumstances by having the ability to change their minds. The authors found the same memory flexibility in T cell decisions enables them to respond robustly to different threats, both to highly virulent pathogens that demand a full immune response, as well as slow-dividing pathogens that seek to simply evade the immune system. “These findings will help us engineer better disease-fighting T cells, that can persist longer in the body to mediate disease clearance,” said Hao Kueh, Bioengineering associate professor.
These studies were performed with UW collaborators Jay Shendure, Junyue Cao, Armita Nourmohammad and Obinna Ukogu in partnership with the lab of Rafi Ahmed at Emory and co-leading author Rajesh Valamparambil from the Ahmed lab.
The research resolve the longstanding uncertainty surrounding how and where memory cells form and opens opportunities for boosting the long-term protective capabilities of the immune system against diverse infectious diseases and cancer.

