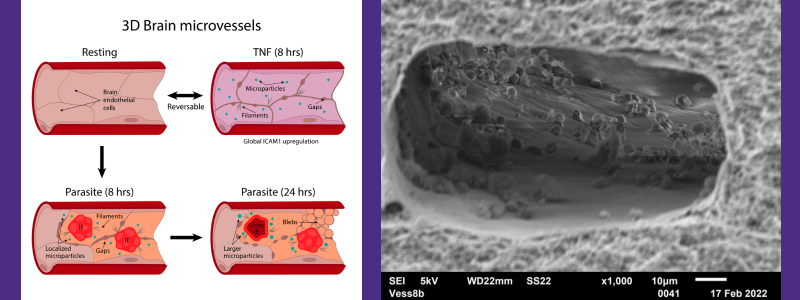
Left: a graphical abstract showing a 3D human brain microvessel platform modeling the spatiotemporal dynamics of cerebral malaria inflammation. Right: a scanning electron microscope image of infected red blood cells binding on 3D perfusable brain microvessels. Image credit: Caitlin Howard
Malaria, a mosquito-borne disease caused by Plasmodium parasites, is a global health concern, with millions of cases reported annually and significant mortality rates. In 2020, there were 241 million cases and over 627,000 deaths attributed to malaria. The most lethal form of malaria is caused by Plasmodium falciparum, responsible for more than 90% of malaria-related deaths. In severe cases, this infection can progress to cerebral malaria, a condition marked by the accumulation of infected red blood cells in the brain’s tiny blood vessels. This process triggers a complex inflammatory response and can be fatal, yet there is still much to learn about its progression.

The cause of cerebral malaria
Plasmodium falciparum parasites have a 48-hour life cycle inside red blood cells. As they mature, they release components that can activate endothelial cells (ECs) lining the blood vessels or compromise the integrity of the blood-brain barrier. This activation of brain ECs can promote the recruitment of white blood cells, increase vessel permeability and even trigger the coagulation cascade – the series of steps that occur during the formation of a blood clot after injury by activating a cascade of proteins called clotting factors.
A 3D microvessel model
The research team explored how the combination of parasite and host inflammatory stimuli affects human brain ECs in cerebral malaria by developing a 3D human brain microvessel model that mimics the brain’s microcirculation. They adapted this model to support the binding, maturation and rupture of P. falciparum-infected red blood cells. By studying how brain blood vessel cells respond to different stages of the parasite’s growth, as well as when exposed to an inflammatory substance called TNF? and white blood cells, the scientists learned about the physical, genetic and functional effects of the parasite and the body’s inflammation in their engineered model.
Research findings
The 3D brain blood vessel model closely resembled what happens in the body, allowing infected cells to attach and grow in place, resulting in a different kind of inflammation compared to what TNF? causes. Through studying the genes, imaging and adding white blood cells, the researchers saw that while TNF? causes inflammation that can be reversed and recruits white blood cells throughout the brain, the parasites caused a different kind of reaction. This reaction included changes in how cells stick together, disruptions in the blood vessel lining and cells dying in certain areas. They also noticed the parasites changed how the vascular cells react to TNF?, preventing the vascular cell recovery and making the inflammation stronger when both are present.
This research shed light on the complex biology of cerebral malaria in the 3D brain microvessel model, suggesting that multiple factors converge to trigger inflammation in the brain’s blood vessels. The ability of the model to support the maturation and rupture of P. falciparum-infected cells, as well as simulate the effects of pro-inflammatory cytokines and white blood cells, provides a valuable in vitro platform for investigating the interactions between brain endothelial cells and the immune system during inflammation.
These findings mark a significant step towards a deeper understanding of malaria, bringing us closer to effective treatments and preventive measures for this devastating disease.
For additional information about the research, visit the Institute for Stem Cell and Regenerative Medicine (ISCRM) website. Ying Zheng is also a faculty member for the sister organization.
The research was made possible with the support of a National Institutes of Health (NIH) grant and a National Science Foundation Graduate Student Research Fellowship.


