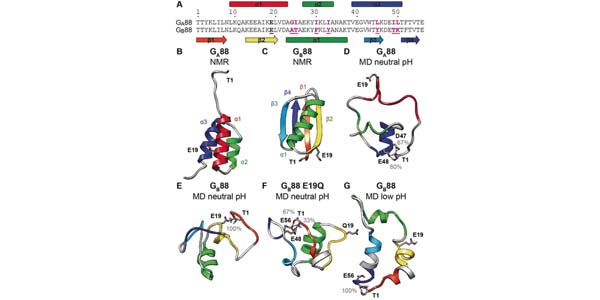
Image: Amino-acid sequence and structural comparison of GA88 and GB88. View full image description
A Carboxylate to Amide Substitution That Switches Protein Folds
Prof. Stefano Gianni, Dr. Michelle E. McCully, Dr. Francesca Malagrinò, Dr. Daniela Bonetti, Dr. Alfonso De?Simone, Prof. Maurizio Brunori, Prof. Valerie Daggett
Angewandte Chemie International Edition, Volume 57, Issue 39 (2018).
Abstract
Metamorphic proteins are biomolecules prone to adopting alternative conformations. Because of this feature, they represent ideal systems to investigate the general rules allowing primary structure to dictate protein topology. A comparative molecular dynamics study was performed on the denatured states of two proteins, sharing nearly identical amino?acid sequences (88?%) but different topologies, namely an all?a?helical bundle protein named GA88 and an a+B?protein named GB88. The analysis allowed successful design of and experimental validation of a site?directed mutant that promotes, at least in part, the switch in folding from GB88 to GA88. The mutated position, in which a glutamic acid was replaced by a glutamine, does not make any intramolecular interactions in the native state of GA88, such that its stabilization can be explained by considering the effects on the denatured state. The results represent a direct demonstration of the role of the denatured state in sculpting native structure.