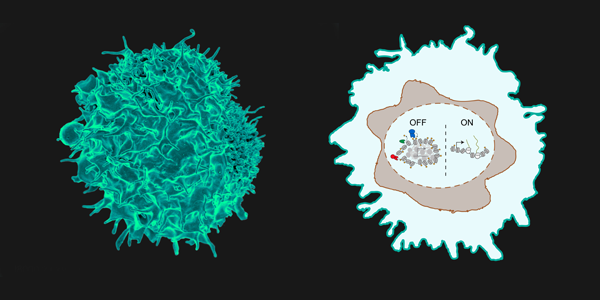
Similar timed gene switches may control other cell fate decisions during development.
In biological development, timing is everything. Different cell types in the embryo must be made at the right times – as well as in the right places – to generate organs and tissues with proper sizes and proportions.
Exactly how cells control the timing of developmental events – especially over timescales that span many cell generations – has been an enduring mystery.

Hao Yuan Kueh, assistant professor of bioengineering and faculty with ISCRM.
Now, new research by Hao Yuan Kueh, assistant professor of bioengineering, and collaborators has uncovered a molecular timer used by stem cells to control when they differentiate, or turn into a specialized cell type. Their research appears March 23 in Cell Reports.
Dr. Kueh and his team study how blood stem cells differentiate into T cells, a specialized type of immune cell that defends the body against viruses and cancer. In this study, they revealed the inner workings of a molecular timer that controls when stem cells commit to becoming a T cell.
To become a T cell, blood stem cells turn on a gene called Bcl11b in response to instructive signals. This gene switches on only 5-10 days after receiving signals – a long time delay – during which cells proliferate and extinguish other fate options before committing to becoming a T cell.
Dr. Kueh and his team have demystified the molecular timer that delays the activation of Bcl11b. This timer involves removal of repressive chemical marks at the Bcl11b gene, together with a physical loosening of the gene itself.
Remarkably, this timed switch generates delays in gene activation that are not only long, but also closely tunable by proteins in the cell. In particular, chromatin-modifying enzymes – proteins that add or remove chemical marks at genes, can speed up or slow down activation in a highly controlled manner.
The gene switch could serve as a modular building block in gene regulatory networks that enable reliable, adjustable control of both an organism’s developmental timing as well as its size and form, the authors suggest.
“Because of their reliable, yet flexible nature, such timed switches likely play widespread roles in controlling timing in development,” says Dr. Kueh. “When acting in dividing progenitors, these switches determine how much progenitor cells proliferate, and ultimately how large a pool of differentiated cells they generate.”
Co-lead authors on the paper are graduate students in the Kueh lab, Nicholas Pease and Sam (Phuc H.B.) Nguyen.
Dr. Kueh’s lab studies how immune cells make fate decisions as they develop from stem cells and as they respond to pathogens and cancer. His work lays the foundation for engineering immune cells to fight cancer and other life-threatening diseases. Dr. Kueh is a member of UW’s Institute for Stem Cell & Regenerative Medicine.
This research was funded by the National Institutes of Health (NIH), including NIH Pathway to Independence Award (Dr. Kueh); National Science Foundation; a Tietze Foundation Stem Cell Scientist Award (Dr. Kueh); and the Institute for Stem Cell and Regenerative Medicine.
Featured art: A colorized scanning electron micrograph of a T cell (left), with a schematic showing a delayed-action gene switch in its nucleus (right). Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health (CC BY-NC-2.0) and UW Kueh Lab