Alzheimer’s disease (AD) is a leading cause of death in the United States, marked by brain cell loss and declining cognitive abilities. One of its hallmarks is the clumping of the amyloid-beta peptide (referred to as Aβ), which leads to the formation of harmful substances that damage brain cells. It was thought this clumping was simply an abnormal process that led to AD. However, University of Washington researchers, led by Valerie Daggett, professor in Bioengineering, suggest that this clumping might not just be a mistake of the body, but rather a response to fight off brain infections.
AD is characterized by a reaction in the brain that causes neuronal inflammation. This inflammation is due to the imbalance between anti-inflammatory and pro-inflammatory signals, resulting in constant activation of certain brain cells called microglia and the release of inflammatory molecules. These microglia usually help clear away Aβ clumps, but when there’s too much, they become overactive, worsening the inflammation, slowing clearance of toxic debris, and contributing to brain cell death.
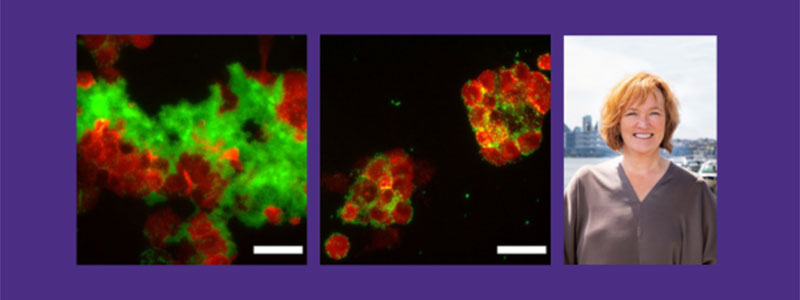
To understand these interactions better, Daggett’s lab conducted experiments to see how bacteria affect the formation of the toxic Aβ clumps in neuronal cells. Using specialized assays researchers can detect the presence of toxic Aβ clumps, referred to as toxic oligomers, and measure their ability to inhibit the bacteria.
We have been able to identify a mechanism of action for the role of Aβ toxic oligomers in innate immunity as a defense against pathogens. This is also the first example of a positive role for the Aβ toxic oligomers. – Valerie Daggett
The research team conducted various experiments with Aβ and Escherichia coli (E. coli) to investigate the effect of bacterial infection on nerve cells and Aβ. They discovered that many bacteria, including E. coli, also form amyloid fibrils, which are incorporated into a biofilm that protects the bacteria, much like what they saw in mammalian proteins and peptides that form amyloid fibrils associated with disease.
The team exposed nerve cells to E. coli and found that the cells produced Aβ toxic oligomers, which then stopped E. coli amyloid fibril formation, destabilizing its protective layer. As a result, the bacteria became more vulnerable to antibiotics. These findings suggest that Aβ might act as a natural defense against infections, but excessive amounts can also harm neurons, a known issue in AD. “We have been able to identify a mechanism of action for the role of Aβ toxic oligomers in innate immunity as a defense against pathogens,” Daggett said. “This is also the first example of a positive role for these toxic oligomers.”