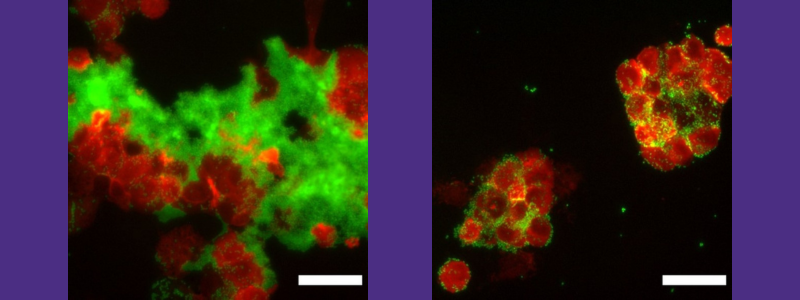
Biofilms protect bacteria from immune system (Biofilm in green and macrophages in red). Left, macrophages prevented from reaching bacteria because of protective biofilm. Right, addition of a-sheet design inhibits amyloid fibril formation, destabilizing biofilm, leading to engulfment of the bacteria by the macrophages
Each year, approximately 35 million adults receive inpatient care in US hospitals. More than half of the patient-days spent in hospitals involve treatment with invasive medical devices. All of these patients are at risk for healthcare-associated infections – the most common adverse event in healthcare delivery worldwide and a significant contributor to mortality and financial losses. In the United States in 2014, approximately one in 25 patients contracted an infection while hospitalized. The frequency of such infections in developing countries is expected to be at least three times higher than that in the United States. Pneumonia and surgical site infections are the most common, followed by gastrointestinal, urinary tract and primary bloodstream infections.
A variety of bacteria are implicated in these infections, and their distribution is dependent on the site of colonization. For example, Staphylococcus aureus dominates surgical site infections, Pseudomonas aeruginosa is a common culprit in pneumonia and Escherichia coli (E. coli) tends to thrive in urinary tract infections. The World Health Organization (WHO) released its “Priority Pathogens List” in February 2017 to guide research and development of new antibiotics, and all three of the “Priority 1 – Critical” bacteria on this list are classified as gram-negative. Gram-negative bacteria are resistant to multiple drugs and are increasingly resistant to most available antibiotics.
WHO has declared that antimicrobial resistance (AMR) is one of the top 10 global public health threats facing humanity. A recent analysis in The Lancet estimates that approximately five million people died due to infections associated with antimicrobial resistance, and approximately 1.3 million deaths are directly attributable to resistance. Six pathogens – E. coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii and Pseudomonas aeruginosa – are responsible for 80% of the deaths.
David and Nancy Auth Endowed Professor of Bioengineering Valerie Daggett and her lab are collaborating with James Bryers, another UW Bioengineering Professor, to overcome, or bypass, the resistance. Their hope is if their compounds effectively target even a fraction of these cases, the effect could be quite impactful in decreasing the death rate from these pathogens.
The Daggett lab is pursuing an innovative approach to bypass antimicrobial resistance by targeting a component of the protective biofilms formed by these bacteria. Many infections are biofilm associated, rendering antibiotic treatments ineffective, leading to additional complications and persistent infections. The lab is focusing on E. coli, which is number one on the Deadly Infections list. The pathogen accounts for the largest proportion of hospital acquired infections in the United States. These infections are a major source of increased costs and treatment complications.
E. coli has developed significant resistance to multiple broad-spectrum antibiotics in recent years, and it is a well-established biofilm former with the ability to produce extracellular amyloid fibrils. These fibrils act as scaffolding for bacterial biofilms, which help the bacteria evade the host immune response and serve as a barrier preventing access of antibiotics to the bacterial cells. In addition, E. coli is a prevalent multi-drug resistant pathogen associated with trauma-related injuries of military personnel.
The Daggett lab’s new paper in Scientific Reports presents studies of inhibition of biofilm formation by E. coli with novel designed peptide-based inhibitors to improve antibiotic efficacy and clearance by the immune system.
This work presents a new, non-antibiotic strategy to fight these infections by inhibiting the formation of amyloid fibrils, which help to stabilize the bacteria’s protective biofilm coating. Daggett hypothesized that, like other amyloid fibrils, the E. coli fibrils contain a unique secondary structure discovered in her group called “a-sheet.” In the paper, results are presented showing the protein that makes up the E. coli fibrils contains a-sheet structure during the early steps of fibril formation. Binding of synthetic a-sheet peptides designed to be complementary to the a-sheet structure in the bacterial protein inhibited fibril formation in live bacterial biofilms. Administration of these a-sheet designs also enhanced antibiotic susceptibility and dispersed biofilm-resident bacteria for improved uptake by immune cells. The ability of synthetic a-sheet peptides to reduce biofilm formation, improve antibiotic susceptibility and enhance clearance by the immune system has broad implications for combating biofilm-associated infections. Learn more about the research in the paper.


