A culture of Belonging in UW Bioengineering
At the University of Washington, diversity, equity, and inclusion are integral to excellence. We value and honor diverse experiences and perspectives, strive to create welcoming and respectful learning environments, and promote access, opportunity, and justice for all.

What Justice, Equity, Diversity and Inclusion means to the UW Bioengineering Community
Towards Justice, we believe that engineers must understand the social justice aspects of technology research and development practices, and are therefore including these topics in our curriculum. Towards Equity, we believe that admissions, hiring and retention practices must utilize best practices shown to overcome institutional and individual biases. Our Department values Diversity as individual differences (e.g., personality, prior knowledge, and life experiences) and group/social differences (e.g., race/ethnicity, class, gender, sexual orientation, country of origin, and ability as well as cultural, political, religious, or other affiliations)1. We seek to have our educational and research programs represent the diversity of our country. Towards Inclusion, the Department focuses on intentionally creating a welcoming environment for everyone, absent of negative feelings and experiences such as fear, insecurity, social tensions, and unaddressed microaggressions, as well as fostering active, intentional, and ongoing engagement with diversity (1,2). These efforts are multi-dimensional and include collaborations with numerous UW programs, recruitment efforts, policies, curriculum, practices, faculty/staff promotions, decision making, and mentoring and continuing education for members of our community.

Justice, Equity, Diversity and Inclusion (JEDI) Committee
The UW Bioengineering JEDI committee has been tasked with developing mechanisms and providing guidance to increase our department’s level of expertise on equity and inclusive teaching and mentoring, and to provide similar expertise to our trainees.
JEDI Resources
Race and Ethnicity
- Graduate Student Equity & Excellence (GSEE)
- Office of Minority Affairs and Diversity (OMA&D)
- UW Samuel E. Kelly Ethnic Cultural Center
- UW Chapter of the National Society of Black Engineers (UW NSBE)
- UW Chapter of the Society of Hispanic Professional Engineers (UW SHPE)
- UW SACNAS Chapter
- w??b?altx? – Intellectual House
- Undocumented student resources
Gender
LGBTQ
Individuals with disabilities
- D Center
- Disability resources for Students
- Disabilities, Opportunities, Internetworking, and Technology
International students
- UW International Student Services (ISS) office
- Undocumented student resources
- Career Center @ Engineering
- Counseling Center
INCLUSIVE ADMISSIONS OR HIRING
INCLUSIVE TEACHING
- PR2ISM
- Teaching@UW
- Equal Access: Universal Design of Instruction | DO-IT
- UW Well-Being for Life and Learning Guidebook
UW INSTITUTIONAL MISSIONS, POLICIES, AND RESOURCES
- UW 2022-2026 Diversity Blueprint
- Diversity at the University of Washington
- Diversity council
- Diversity statistics & policies
- Office of Inclusive Excellence in COE
- Strategic planning in the College of Engineering
- Disability Services
Feedback & Reporting Mechanisms
It is our goal that all members of the BIOE community feel included and supported. We want to highlight the resources available to you if you would like to provide feedback to improve the program or resolve a situation, or would like support in an incident of bias. We have provided links to different methods of providing feedback or reporting, and some information to help you decide which suits your purpose.
See also
Diversity at the University of Washington
UW Equity Focus, the UW’s hub for stories highlighting diversity and equity
In the News
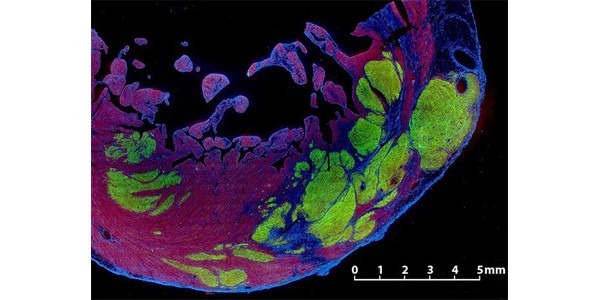
Stem cells restore working heart muscle in monkeys after heart failure
Monkeys with heart failure regrew working heart muscle after receiving human stem cells, report a UW team led by Charles Murry, professor of bioengineering, pathology and cardiology/medicine.
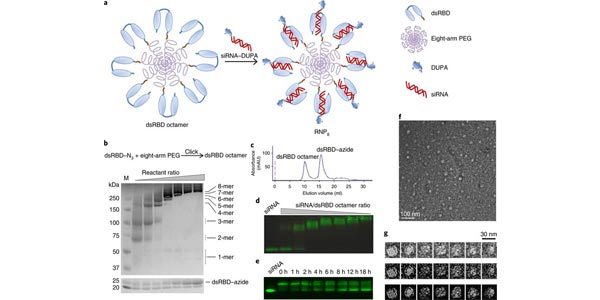
A ribonucleoprotein octamer for targeted siRNA delivery
Professor Xiaohu Gao and colleagues have created a new way to target prostate tumors that overcomes past challenges of designing effective drug delivery methods. This versatile nanocarrier design should offer opportunities for the clinical translation of therapies based on intracellularly acting biologics.
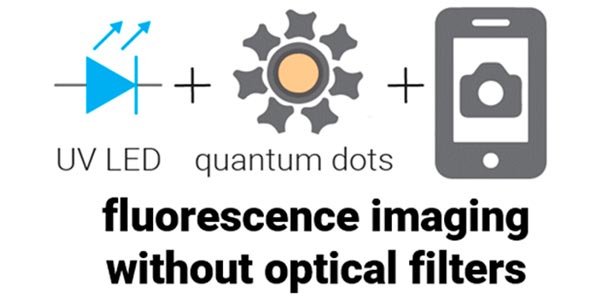
Mobile Phone Ratiometric Imaging Enables Highly Sensitive Fluorescence Lateral Flow Immunoassays without External Optical Filters
Professor Paul Yager's lab has created a method that enables optical-filter free mobile imaging for medical diagnostics, a first step towards enabling a new generation of highly sensitive, point-of-care fluorescence assays.
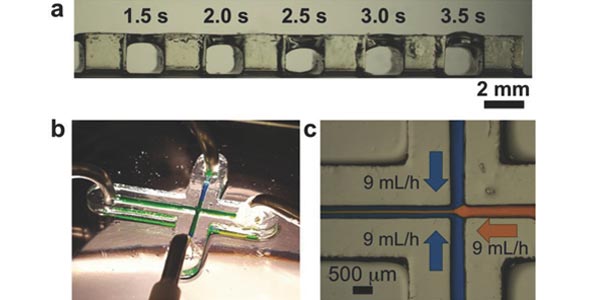
Desktop-Stereolithography 3D-Printing of a Poly(dimethylsiloxane)-Based Material with Sylgard-184 Properties
Professor Albert Folch's lab reports on the formulation, characterization, and SL application of a 3D?printable PDMS resin (3DP?PDMS) based on commercially available PDMS?methacrylate macromers, a high?efficiency photoinitiator and a high?absorbance photosensitizer. 3DP?PDMS resin enables assembly?free, automated, digital manufacturing of PDMS, which should facilitate the prototyping of devices for microfluidics, organ?on?chip platforms, soft robotics, flexible electronics, and sensors, among others.
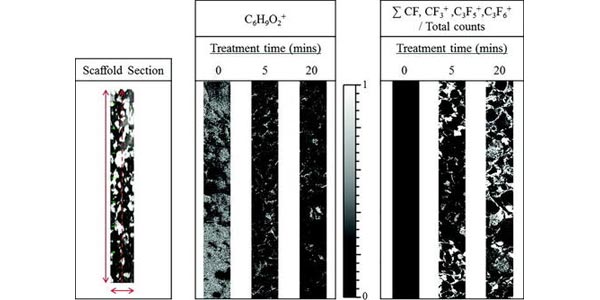
Time of flight secondary ion mass spectrometry—A method to evaluate plasma-modified three-dimensional scaffold chemistry
Research Associate Professor Lara Gamble and colleagues report on a technique for characterizing the distribution and composition of chemical species through complex porous scaffolds. This approach could be widely applicable for ToF-SIMS analysis of scaffolds modified by multiple plasma processing techniques as well as alternative surface modification approaches.
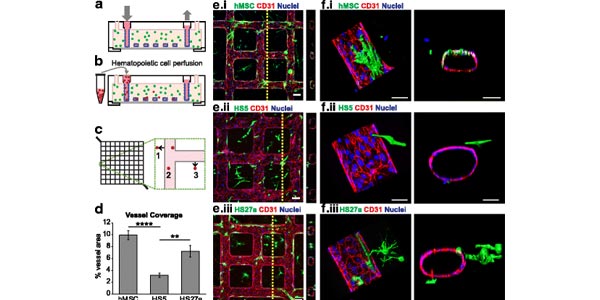
Engineering a multicellular vascular niche to model hematopoietic cell trafficking
Assistant Professor Ying Zheng and colleagues developed an engineered human vascular marrow niche to examine the three-dimensional cell interactions that direct hematopoietic cell trafficking. The platform provides a tool to advance study of the interactions between endothelial cells, marrow-derived fibroblasts and hematopoeitic cells that comprise the marrow vascular niche, and has potential for use in testing therapeutics and personalized medicine.