Nathan R Chan, Billanna Hwang, Buddy D Ratner, James D Bryers
Journal of Tissue Engineering and Regenerative Medicine, 2022 March; 16(3):297-310.
DOI: 10.1002/term.3280. Epub: 2022 Jan 4.
Abstract
Porous precision-templated scaffolds (PTS) with uniformly distributed 40 µm spherical pores have shown a remarkable ability in immunomodulating resident cells for tissue regeneration. While the pore size mediated pro-healing response observed only in 40 µm pore PTS has been attributed to selective macrophage polarization, monocyte recruitment and phenotype have largely been uncharacterized in regulating implant outcome. Here, we employ a double transgenic mouse model for myeloid characterization and a multifaceted phenotyping approach to quantify monocyte dynamics within subcutaneously implanted PTS. Within 40 µm PTS, myeloid cells were found to preferentially infiltrate into the scaffold. Additionally, macrophage receptor with collagenous structure (MARCO), an innate activation marker, was significantly upregulated within 40 µm PTS. When 40 µm PTS were implanted in monocyte-depleted mice, the transcription of MARCO was significantly decreased and an increase in pro-inflammatory inducible nitric oxide synthase (iNOS) and tumor necrosis factor alpha (TNF?) were observed. Typical of a foreign body response (FBR), 100 µm PTS significantly upregulated pro-inflammatory iNOS, secreted higher amounts of TNF?, and displayed a pore size dependent morphology compared to 40 µm PTS. Overall, these results identify a pore size dependent modulation of circulating monocytes and implicates MARCO expression as a defining subset of monocytes that appears to be responsible for regulating a pro-healing host response.
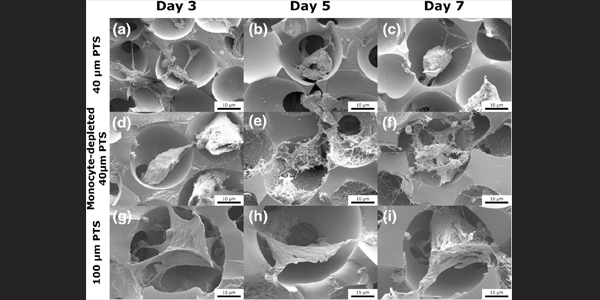
Image caption: Scanning electron microscopy images of subcutaneously implanted scaffolds in LysM-Cre+/0:mT/mG+/0 mice at Day 3, Day 5, and Day 7 post-implantation. (a–c) 40 µm precision-templated scaffolds (PTS) in untreated mice, (d–f) 40 µm PTS in monocyte-depleted mice, and (g–i) 100 µm PTS in untreated mice