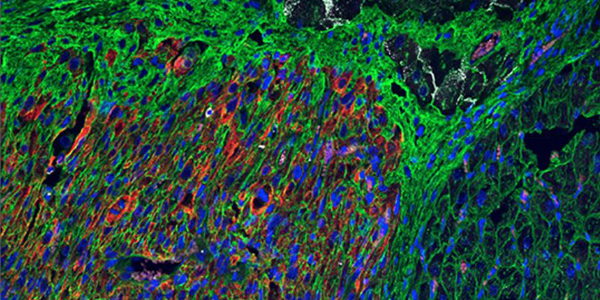
Image: An hPSC-cardiomyocyte graft 2 weeks post-transplantation, marked by human-specific slow skeletal cardiac troponin I (ssTnI) (red) (Bedada et al., 2014), interacts with Cx40-positive (white) Purkinje fibers. Wheat germ agglutinin (WGA) (green) delineates cardiac sarcolemma. Purkinje-transitional cell-graft and direct Purkinje-graft interactions are observed.
Kenta Nakamura, Lauren E. Neidig, Xiulan Yang, Gerhard J. Weber, Danny El-Nachef, Hiroshi Tsuchida, Sarah Dupras, Faith A. Kalucki, Anu Jayabalu, Akiko Futakuchi-Tsuchida, Daisy S. Nakamura, Silvia Marchianò, Alessandro Bertero, Melissa R. Robinson, Kevin Cain, Dale Whittington, Rong Tian, Hans Reinecke, Lil Pabon, Björn C. Knollmann, Steven Kattman, R. Scott Thies, W. Robb MacLellan, Charles E. Murry
Stem Cell Reports VOLUME 16, ISSUE 10, P2473-2487, OCTOBER 12, 2021; DOI:https://doi.org/10.1016/j.stemcr.2021.08.005
Abstract
Heart failure remains a significant cause of morbidity and mortality following myocardial infarction. Cardiac remuscularization with transplantation of human pluripotent stem cell-derived cardiomyocytes is a promising preclinical therapy to restore function. Recent large animal data, however, have revealed a significant risk of engraftment arrhythmia (EA). Although transient, the risk posed by EA presents a barrier to clinical translation. We hypothesized that clinically approved antiarrhythmic drugs can prevent EA-related mortality as well as suppress tachycardia and arrhythmia burden. This study uses a porcine model to provide proof-of-concept evidence that a combination of amiodarone and ivabradine can effectively suppress EA. None of the nine treated subjects experienced the primary endpoint of cardiac death, unstable EA, or heart failure compared with five out of eight (62.5%) in the control cohort (hazard ratio = 0.00; 95% confidence interval: 0–0.297; p = 0.002). Pharmacologic treatment of EA may be a viable strategy to improve safety and allow further clinical development of cardiac remuscularization therapy.