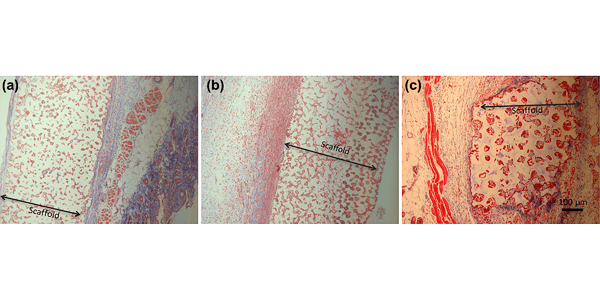
Image: Masson’s trichrome stain of tissue sample after 3 weeks implantation. (a) 40-µm PDMS scaffold; (b) 40-µm pHEMA scaffold; (c) 90-µm PDMS scaffold. Scale Bar?=?100??m
Precision-porous templated scaffolds of varying pore size drive dendritic cell activation
, , , . Precision-porous templated scaffolds of varying pore size drive dendritic cell activation. Biotechnology and Bioengineering. 2018;115:1086–1095.
Abstract
Scaffold based systems have shown significant potential in modulating immune responses in vivo. While there has been much attention on macrophage interactions with tissue engineered scaffolds for tissue regeneration, fewer studies have looked at the effects of scaffold design on the response of immune cells?that is, dendritic cells (DCs). Here, we present the effects of varying pore size of poly (2-hydroxyethyl methacrylate) (pHEMA) and poly(dimethylsiloxane) (PDMS, silicone) scaffolds on the maturation and in vivo enrichment of DCs. We employ a precision templating method to make 3-D porous polymer scaffolds with uniformly defined and adjustable architecture. Hydrophilic pHEMA and hydrophobic PDMS scaffolds were fabricated in three pore sizes (20, 40, 90??m) to quantify scaffold pore size effects on DCs activation/maturation in vitro and in vivo. In vitro results showed that both pHEMA and PDMS scaffolds could promote maturation in the DC cell line, JAWSII, that resembled lipopolysaccharide (LPS)-activated/matured DCs (mDCs). Scaffolds with smaller pore sizes correlate with higher DC maturation, regardless of the polymer used. In vivo, when implanted subcutaneously in C57BL/6J mice, scaffolds with smaller pore sizes also demonstrated more DCs recruitment and more sustained activation. Without the use of DC chemo-attractants or chemical adjuvants, our results suggested that DC maturation and scaffold infiltration profile can be modulated by simply altering the pore size of the scaffolds.