Assistant Professor
pmjboyle@uw.edu
Phone: (206) 685-1392
Office: Foege N310H (main campus); Brotman 311 (SLU)
Patrick M. Boyle
- Predicting future health risks in patients with embolic stroke of undetermined source
- Optimizing treatment of atrial rhythm disorders via personalized simulations
- Conceptualizing next-gen cardiac devices that harness new tech like optogenetics
- Investigating new applications of heart-like cells induced from patient-derived stem cells
- Exploiting AI to predict lethal cardiovascular events, including in patients with COVID-19
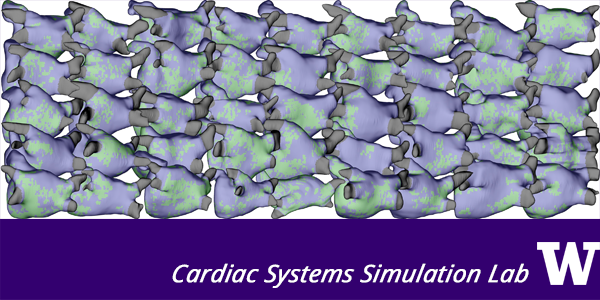
The Cardiac Systems Simulation (CardSS) Lab uses models of the human heart to cultivate knowledge about arrhythmia mechanisms and concoct new treatment strategies for these conditions. These problems are part of an enormous global health concern, especially since heart rhythm disorders are becoming more prevalent as the number of elderly individuals continues to grow. We are developing approaches to identify those with future risk of complications (sudden cardiac arrest, embolic stroke of undetermined source, worsening arrhythmia) via simulations in models reconstructed from MRI scans. We are also using computational tools to lower barriers to translation in regenerative medicine via injection of heart-like cells derived from a patient’s own stem cells. The same modeling techniques can help us envision and text radical new biomedical devices like light-based defibrillators. Our most recent research thrust uses artificial intelligence and machine learning to tease information out of standard EKG signals. This is particularly noteworthy in the context of COVID-19, since we can help front-line care teams predict risk for heart complications while reducing the need for daily measurements. Finally, we have an overarching interest in driving technological development to accelerate and simplify the cardiac modeling process.
Our work is highly interdisciplinary and often involves frequent and intense interaction with collaborators including high performance computing experts, wet lab biologists, optics researchers, medical imaging specialists, and cardiologists conducting procedures to treat arrhythmia in patients. Our trainees are deeply embedded in translational research and attend weekly clinical meetings with the full UW Medicine cardiac electrophysiology team, where they are exposed to the same high-level content learned by fellows-in-training.
Ph.D., Biomedical Engineering, University of Calgary, 2011
B.Sc. with Distinction, Internship program, Computer Engineering, University of Calgary, 2005
Assistant Research Professor, Department of Biomedical Engineering and Institute for Computational Medicine, Johns Hopkins University, 2015-18
Assistant Research Scientist, Institute for Computational Medicine, Johns Hopkins University, 2014-15
NSERC Postdoctoral Fellow, Computational Cardiology Lab, Institute for Computational Medicine, Johns Hopkins University, 2011-14
UW Population Health Initiative, COVID-19 Rapid Response Grant, 2020
American Heart Association Scientist Development Grant, 2016-19
Appointed as a Fellow of the Heart Rhythm Society (FHRS designation), 2017
Special Selection: Heart Rhythm Society EP Concepts Ignited Session, 2017
Licensed as a Canadian Professional Engineer (P.Eng. designation), 2013
Cardiac Physiome Workshop, Outstanding Scientific Poster Presentation, 2012
Computing in Cardiology Conference, Rosanna Degani Young Investigator Award (Finalist), 2012
NSERC Post-Doctoral Fellowship, 2011-13
AStech Foundation Leaders of Tomorrow (Honouree), 2011
NSERC Post-Graduate Scholarship D, 2008-10
NSERC Post-Graduate Scholarship M, 2005-07
BIOEN400: Fundamentals of Bioengineering Design [link]
View all publications:
Google Scholar
PubMed
* Equal co-author contributions
Boyle PM*, Zghaib T*, et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng. 2019 Nov;3(11):870-879. PMID: 31427780.
Boyle PM*, Franceschi WH*, et al. New insights on the cardiac safety factor: Unraveling the relationship between conduction velocity and robustness of propagation. J Mol Cell Cardiol. 2019 Mar;128:117-128. PMID: 30677394.
Boyle PM, Karathanos TV, Trayanova NA. Cardiac Optogenetics: 2018 (Invited State-of-the-art Review). JACC Clin Electrophysiol. 2018 Feb;4(2):155-167. PMID: 29749932
Boyle PM, et al. Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation. Front Physiol. 2018 Apr 19;9:414. PMID: 29725307
Boyle PM, et al. Termination of re-entrant atrial tachycardia via optogenetic stimulation with optimized spatial targeting: insights from computational models. J Physiol. 2018 Jan 15;596(2):181-196. PMID: 29193078
Deng D*, Murphy MJ*, […], and Boyle PM. Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate. Chaos. 2017 Sep;27(9):093932. PMID: 28964164
Bruegmann T*, Boyle PM*, et al. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J Clin Invest. 2016 Oct 3;126(10):3894-3904. PMID: 27617859
Zahid S*, Cochet H*, Boyle PM*, et al. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res. 2016 Jun 1;110(3):443-54. PMID: 27056895
Boyle PM*, Park CJ*, et al. Sodium current reduction unmasks a structure-dependent substrate for arrhythmogenesis in the normal ventricles. PLoS One. 2014 Jan 28;9(1):e86947. PMID: 24489810
Boyle PM et al. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat Commun. 2013;4:2370. PMID: 23982300
Boyle PM et al. Transmural IK(ATP) heterogeneity as a determinant of activation rate gradient during early ventricular fibrillation: mechanistic insights from rabbit ventricular models. Heart Rhythm. 2013 Nov;10(11):1710-7. PMID: 23948344
Boyle PM et al. Fusion during entrainment of orthodromic reciprocating tachycardia is enhanced for basal pacing sites but diminished when pacing near Purkinje system end points. Heart Rhythm. 2013 Mar;10(3):444-51. PMID: 23207137
Boyle PM and Vigmond EJ. An intuitive safety factor for cardiac propagation. Biophys J. 2010 Jun 16;98(12):L57-9. PMID: 20550885
Boyle PM et al. Purkinje-mediated effects in the response of quiescent ventricles to defibrillation shocks. Ann Biomed Eng. 2010 Feb;38(2):456-68. PMID: 19876737
In the News
Patrick Boyle honored with CMBE Young Innovator Award
2023-09-28T06:18:48-07:00September 21st, 2023|
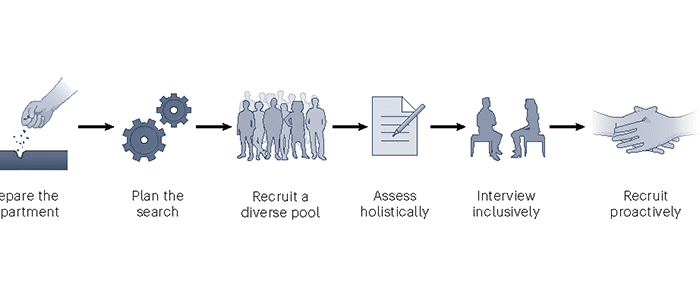
UW bioengineering researchers help create a roadmap to diversify faculty hiring
2023-08-16T05:50:45-07:00August 16th, 2023|
Boyle lab awarded $2.9M to create new techniques to assess stroke risk
2022-07-18T16:02:34-07:00June 28th, 2022|
Computational modeling identifies embolic stroke of undetermined source patients with potential arrhythmic substrate
2021-05-04T22:24:11-07:00May 4th, 2021|
Study yields new clue to strokes of undetermined source
2021-05-04T22:10:30-07:00May 4th, 2021|