Joint Professor of Bioengineering and Chemical Engineering
Michael L. & Myrna Darland Endowed Chair in Technology Commercialization
ratner@uw.edu
Phone: (206) 685-1005
Office: Foege N330J
Buddy D. Ratner
Center for Dialysis Innovation: https://cdi.washington.edu/
UWEB Industry Consortium: https://sites.uw.edu/uweb21/
Surface analysis by ESCA, SIMS, STM, FTIR-ATR, AFM
Plasma deposition of thin films
Proteins at interfaces
Healing, regeneration, tissue engineering, macrophages
Kidney dialysis, blood compatibility
We engineer new biomaterial surfaces using a wide range of technologies. For example, radio-frequency plasma deposition (a method borrowed from microelectronics) can readily place interesting thin films on existing medical device surfaces. These films can be used in the precision immobilization of key signaling molecules. We also synthesize new polymers that can be biostable, environmentally responsive, biodegradable and/or porous (i.e., scaffolds). The new surfaces and materials made in our laboratory are studied in contact with proteins, blood, living cells and tissues (in vivo and in vitro).
Recently, there has been considerable interest in tissue engineering in my laboratory. Tissue engineering exploits all the above principles in the context of tissue and organ reconstruction and regeneration. Specific tissue engineering projects in the Ratner lab have aimed toward heart muscle, esophagus, bone, cartilage, bladder, vagina and cornea. Another new project seeks to model cancer tumor microenvironments using tissue engineering ideas.
Biomaterials/biocompatibility projects ongoing in my laboratory include:
- drug delivery devices
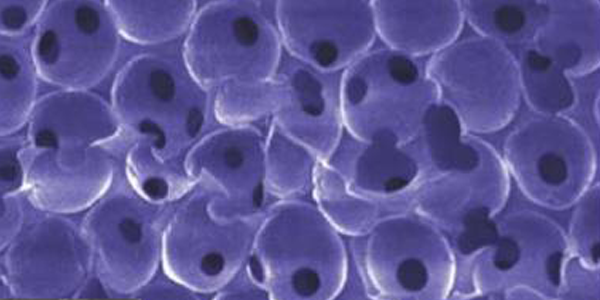
- porous scaffolds
- tissue engineering
- angiogenesis
- healing in soft tissue
- bioelectrode performance
- bioattachment
- biorecognition
- polyurethanes
- hydrogels
- biodegradable polymers
- non-fouling surfaces
- blood-contacting materials
- bacterial biofilms/infection
Biomaterial surfaces are the only part of a biomaterial or medical device that is seen by the body. Surfaces present unique analytical problems because of the small mass of material involved (a billionth of a gram of matter per square centimeter is typical). Special instruments are required to study surfaces, and we adapt methods developed in the physics and microelectronics communities to problems in biology and medicine. We use electron spectroscopy for chemical analysis (ESCA), secondary ion mass spectrometry (SIMS), infrared spectroscopy, scanning probe microscopies, surface plasmon resonance and sum frequency generation to observe surface structure and biological interactions.
In 2016, we launched the Center for Kidney Dialysis (CDI). CDI aims to address many of problems in kidney dialysis that shorten the lives of patients including blood compatibility, fibrosis, restenosis, bacterial infection, membrane limitations, etc. I serve as co-director of CDI along with Professor Jonathan Himmelfarb (MD nephrologist).
BS, Chemistry, Brooklyn College, 1967
2016 Kammermeyer Lecturer, University of Iowa
2015 Distinguished Service Award, ACS Division of Polymer Chemistry
2015 Langmuir Lecture, American Chemical Society (COLL Division)
2015 Most Cited Paper Award, Annals of Biomedical Engineering
2014 Fellow, Polymer Division of American Chemical Society
2014 University of Washington School of Medicine 2014 Lifetime Innovator and Inventor Award
2011-2012 AVS Biointerphases Lectureship
2010-2012 UW Entrepreneurial Faculty Fellow
2012 George Winter Award, European Society for Biomaterials
2012 Honorary Professor of Sichuan University
2012 Journal of Materials Science-Materials in Medicine “Best Paper Published in 2011”
2012 Fellow, Tissue Engineering and Regenerative Medicine International Society (FTERM)
2011 Pierre Galletti Award of the American Institute of Medical & Biological Engineering
2011 Fellow, American Chemical Society
2010 McGowan Distinguished Lecturer, University of Pittsburgh
2010 Annual Faculty Lecturer, University of Washington
2009 Chandra P. Sharma Award, Society for Biomaterials & Artificial Organs (India)
2009 Acta Biomaterialia Gold Medal Award
2008 BMES Pritzker Distinguished Lecturer Award
2008 J. Edward Berk Lecture Medal
2008 Listed in AIChE’s “One Hundred Chemical Engineers of the Modern Era”
2008 Frontiers of Science Award, Society of Cosmetic Chemists
2008 Kewaunee Lecturer, Duke University
2007 Bayer Lectureship, University of Akron
2006 C. William Hall Award, Society for Biomaterials
2004 Founders Award, Society for Biomaterials
2004 Distinguished Lecturer, University of Utah
2002 Elected to the National Academy of Engineering of the United States of America
2002 Medard W. Welch Award, American Vacuum Society
2002 Chair, Roundtable on Biomedical Engineering Materials and Applications (BEMA)
2000 Science In Medicine Lecturer, University of Washington
2000 Joe Smith Distinguished Lecturer, University of California, Davis
2004-2005 Robert F. Rushmer Professor of Bioengineering, University of Washington
2001-2004 Washington Research Foundation Endowed Professor of Bioengineering
1999 American Vacuum Society Distinguished Lecturer
1998 C.M.A. Stine Award for Materials Science, AIChE
1996 Van Ness Lecturer, Rensselear Polytechnic Institute
1995 Chair, Gordon Research Conference on Biocompatibility & Biomaterials, July 23-28
1993 Founding Fellow of the American Institute of Medical and Biological Engineering (AIMBE)
1993 Fellow, American Vacuum Society
1993 Fellow, Society for Biomaterials
1991 Perkin Elmer Physical Electronics Award for Excellence in Surface Science
1990 Burlington Resources Foundation Faculty Achievement Award for Outstanding Research
1988 Clemson Award for Contributions to the Literature
BIOEN 492/592: Surface Analysis
BIOEN 490: Biomaterials
BIOEN 504: Introduction to Technology Commercialization
BIOEN 505: Biomedical Entrepreneurship
BIOEN 511: Biomaterials Seminar
“The Biocompatibility Manifesto: Biocompatibility for the Twenty-first Century,” B.D. Ratner, J. Cardiovasc. Transl. Res., 4(5):523-7, 2011. PMID: 21710333
“Quantifying the effect of pore size and surface treatment on epidermal incorporation into percutaneously implanted sphere-templated porous biomaterials in mice,” R.A, Underwood, M.L. Usui, G. Zhao, K.D. Hauch, M.M. Takeno, B.D. Ratner, A.J. Marshall, X. Shi, J.E. Olerud, P. Fleckman, J. Biomed. Mater. Res. A, 98(4):499-508, 2011. PMID: 21681942
“Identifying Individual Cell Types in Heterogeneous Cultures Using Secondary Ion Mass Spectrometry Imaging with C60 Etching and Multivariate Analysis,” C.A. Barnes, J. Brison, M. Robinson, D.J. Graham, D.G. Castner, B.D. Ratner, Analytical Chemistry, 84(2):893-900, 2012. PMID: 22098081 PMCID: PMC3264684
“Plasma Pencil Atmospheric Mass Spectrometry Detection of Positive Ions from Micronutrients Emitted from Surfaces,” M.J. Stein, E. Lo, D.G. Castner, B.D.Ratner, Analytical Chemistry, 84(3):1572-8, 2012. PMID: 22243439 PMCID: PMC3282486
“Amphiphilic Self-assembled ‘Polymeric Drugs.’ Morphology, Properties and Biological Behaviour of Nanoparticles,” M.L. Lopez-Donaire, E. Sussman, M. Fernandez-Gutierrez, A. Mendez-Vilas, B. D. Ratner, B. Vazquez-Lasa, J. San Ramon, Biomacromolecules, 13(3):624-35, 2012. PMID: 22339281
“Cutaneous and inflammatory response to long-term percutaneous implants of sphere-templated porous/solid poly(HEMA)and silicone in mice,” P. Fleckman, M. Usui, G.A. Zhao, R. Underwood, M. Maginness, A. Marshall, C. Glaister, B. Ratner, J. Olerud, J.Biomed. Mater. Res.A, 100(5):1256-68, 2012. PMID: 22359383 PMCID: PMC3506026
“Macrophage Polarization: An Opportunity for Improved Outcomes in Biomaterials and Regenerative Medicine, B.N. Brown, B.D. Ratner, S.B. Goodman, S. Amar, S.F. Badylak, Biomaterials, 33(15):3792-802, 2012. PMID: 22386919 PMCID: PMC3727238
“Engineering biomaterials to integrate and heal: The biocompatibility paradigm shifts,” J.D. Bryers, C.M. Giachelli, B.D. Ratner, Biotechnology and Bioengineering, 109(8):1898-911, 2012. PMID: 22592568 PMCID: PMC3490630
“The effect of octadecyl chain immobilization on the hemocompatibility of poly (2-hydroxyethyl methacrylate),” M. Fischer, C.P. Baptista, I.C. Gonçalves, B.D. Ratner, C. Sperling, C. Werner, M.C.L. Martins, M.A. Barbosa, Biomaterials, 33(31):7677-85, 2012. PMID: 22840226
“Integrated Bi-Layered Scaffold for Osteochondral Tissue Engineering,” A. Galperin, R.A. Oldinski, S.J. Florczyk, J.D. Bryers, Miqin Zhang, B.D. Ratner, Advanced Healthcare Materials, 2(6):872-83, 2012. PMID:23225568 PMCID: PMC3644393
“Synthesis and Fabrication of a Degradable Poly(N-isopropyl acrylamide) Scaffold for Tissue Engineering Applications,” A. Galperin, T.J. Long, S. Garty, B.D. Ratner, J. Biomed. Mater. Res. A, 101(3):775-86, 2013. PMID: 22961921 PMCID: PMC3712632
“Capillary Force Seeding of Sphere-Templated Hydrogels for Tissue-Engineered Prostate Cancer Xenografts,” T.J. Long, M. Takeno, C.C. Sprenger, S.R. Plymate, B.D. Ratner, Tissue Eng Part C Methods, 19(9):738-44, 2013. PMID:23373788
“Prevascularized Microtemplated Fibrin Scaffolds for Cardiac Tissue Engineering Applications,” K.S, Thomson, F.S. Korte, C.M. Giachelli, B.D. Ratner, M. Regnier, M. Sctena, Tissue Engineering Part A, 19(7-8):967-77, 2013. PMID: 23317311 PMCID: PMC3589898
“Zwitterionic hydrogels implanted in mice resist the foreign-body reaction,” L. Zhang, Z. Cao, T. Bai, L. Carr, J-R Ella-Menye, C. Irvin, B.D. Ratner, S. Jiang, Nature Biotechnology, 31(6):553-6, 2013. PMID: 23666011
“Going out on a limb about regrowing an arm,” B.D. Ratner, J. Materials Sci: Materials in Medicine, 24(11):2645-9, 2013. PMID: 24132739
“Engineered Biomaterials Control Differentiation and Proliferation of Human-Embryonic-Stem-Cell-Derived Cardiomyocytes via Timed Notch Activation,” J.C. Tung, S.L. Paige, B.D. Ratner, C.E. Murry, C.M. Giachelli, Stem Cell Reports, 2(3):271-81, 2014. PMID: 24672751 PMCID: PMC3964284
“Digital Drug Delivery: On-Off Ultrasound Controlled Antibiotic Release from Coated Matrices with Negligible Background Leaching,” M.L. Noble, P.D. Mourad, B.D. Ratner, Biomater. Sci., 2(6):893-902, 2014.
“Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction,” E.M. Sussman, M.C. Halpin, J. Muster, R.T. Moon, B.D. Ratner, Annals of Biomedical Engineering, 42(7):1508-16, 2014. PMID: 24248559
“Prostate cancer xenografts engineered from 3D precision-porous poly(2-hydroxyethyl methacrylate) hydrogels as models for tumorigenesis and dormancy escape,” T.J. Long, C.C. Sprenger, S.R. Plymate, B.D. Ratner, Biomaterials, 35(28):8164-74, 2014. PMID: 24942815
“A tough, precision-porous hydrogel scaffold: Ophthalmologic applications,” W.Q. Teng, T. Long, T. Shen, B. Ratner, Biomaterials, 35(32): 8916-8926, 2014.
“Revealing cytokine-induced changes in the extracellular matrix with secondary ion mass spectrometry,” A. J. Taylor, B. D. Ratner, L.D.K. Buttery, M. R. Alexander, Acta Biomaterialia, 14:70-83, 2014.
“Healing with medical implants: The body battles back,” B. D. Ratner, Sci. Transl. Med. 7, 272fs4, 2015.
“Drug Encapsulated Polymeric Microspheres for Intracranial Tumor Therapy: A Review of the Literature” J. A. Floyd, A. Galperin, B. D. Ratner, Advanced Drug Delivery Reviews, 91, 23–37 2015
“A Precision-Porous PolyHEMA-Based Scaffold as an Antibiotic-Releasing Insert for a Scleral Bandage” A. Galperin, K. Smith, N. Geisler, J. Bryers, B. Ratner, ACS Biomaterials Science & Engineering, 1, 593?600 2015
“Drug Encapsulated Aerosolized Microspheres as a Biodegradable, Intelligent Glioma Therapy” (authors: J. A. Floyd, B.D. Ratner, Journal of Biomedical Materials Research A, 104A, 544–552, 2016
“Blood Compatibility Assessment of Polymers used in Drug Eluting Stent Coatings,” Luisa Mayorga Szott, Colleen A. Irvin, Mikael Trollsas, Syed Hossainy, Buddy D. Ratner, Biointerphases, 11. 029806, 2016
“A Pore Way to Heal and Regenerate: 21st Century Thinking on Biocompatibility” B. D. Ratner, Regenerative Biomaterials, 2016, doi: 10.1093/rb/rbw006 (invited, refereed perspective article)
In the News
2023 Hoffman Lecture – Buddy Ratner
2023-10-12T09:43:51-07:00September 28th, 2023|
Two BioE undergrads receive Population Health Recognition Awards
2022-06-02T23:27:46-07:00June 2nd, 2022|
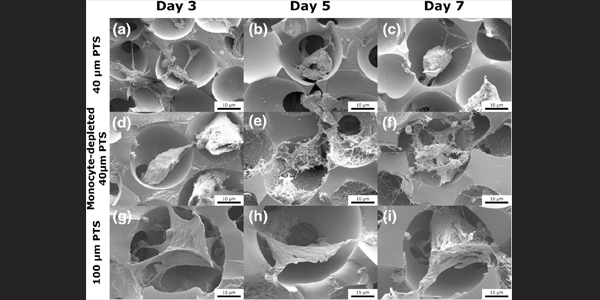
Monocytes contribute to a pro-healing response in 40 µm diameter uniform-pore, precision-templated scaffolds
2022-04-19T10:51:58-07:00April 19th, 2022|
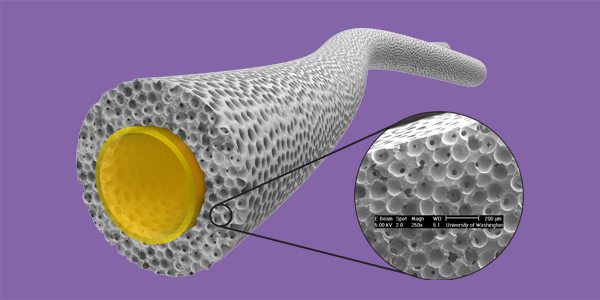
Precision-engineered porous, flexible grafts promote healing, reduce scarring
2021-12-16T13:51:59-08:00December 15th, 2021|
Ratner, UW team win prize for artificial kidney system
2021-09-28T16:43:01-07:00September 28th, 2021|
Buddy Ratner wins Bioelastomer Award
2021-05-11T10:42:43-07:00November 23rd, 2020|