A number of UW Bioengineering faculty members quickly pivoted and are adapting their research to addressing the needs created by the coronavirus pandemic. From developing rapid at-home tests to vaccines and treatments, here is a sampling of some of the ways UW BioE faculty, staff and students are stepping up to help.
 Using A.I. to predict risk of heart complications from COVID-19
Using A.I. to predict risk of heart complications from COVID-19
Patrick Boyle, assistant professor, and his collaborators in UW cardiology and epidemiology are developing a way to use artificial intelligence to help frontline health care workers predict which COVID-19 patients are at highest risk for heart complications from the illness. Read the full story.
 Developing biomaterials for self-vaccination
Developing biomaterials for self-vaccination
James Bryers, professor: The Bryers lab is developing biomaterials that can self-vaccinate the host, using a novel vaccine delivery approach. To combat the SARS-CoV-2 virus, the researchers are adapting their work on biodegradable hydrogels that spontaneously form a porous scaffold upon injection, that then enhances the adaptive immune response upon delivering a self-replicating mRNA vaccine.
 Bioengineering Students Developing Platform to Assess Public Understanding of COVID-19
Bioengineering Students Developing Platform to Assess Public Understanding of COVID-19
COVIDIA: A group of UW Bioengineering undergraduates came together this spring to develop a way to promote public understanding of the COVID-19 crisis. Through two design challenge competitions and the aid of mentors, they have refined their idea and are developing a platform called COVIDIA that aims to capture how well the public understands the disease and identify gaps in knowledge.
3D print of a SARS-CoV-2 spike protein. Credit: NIH
Locking up the spike protein to prevent infection
Gianluca Interlandi, research assistant professor, and Wendy Thomas, professor: Antibody Dependent Enhancement, a type of immune system backfiring that causes increased infection in cells, is likely responsible for disappointing results on experimental vaccines for SARS and MERS, and possibly for the sudden onset of lethal complications in otherwise recovering COVID-19 patients. The Interlandi and Thomas team is designing and characterizing a SARS-CoV-2 spike protein (the major protein the virus uses to invade human cells by changing shape, or “conformation” to fuse with the cell membrane) that is locked in an inactive conformation. If the spike protein is locked in an inactive shape, it can’t infect a cell. This was a key step in the team’s success in overcoming a similar issue with vaccine development for the bacterial adhesin FimH that they studied and undergoes similar conformational changes.
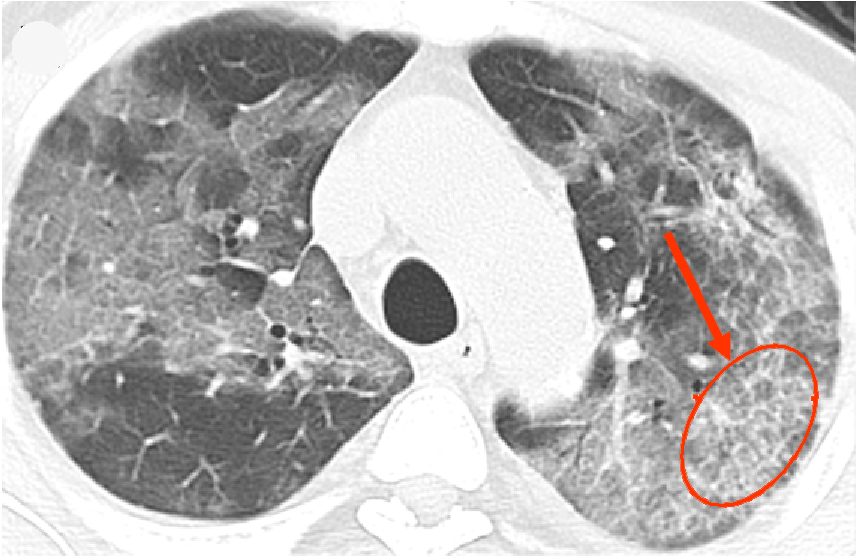
CT scan of lung with COVID-19. Credit: Kinahan Lab
Creating a national COVID-19 image repository
Paul Kinahan, joint professor of radiology and bioengineering: Dr. Kinahan is part of a group of national medical imaging societies that is creating and preparing to launch a COVID-19 national image repository at the National Institute of Biomedical Imaging and Bioengineering (NIBIB), part of the National Institutes of Health.

Enos Kline, Lutz Lab. Mark Stone/UW Photography
Meeting the need for COVID-19 test kits: pivoting from Seattle Flu Study and developing new rapid tests
Barry Lutz, associate professor: Dr. Lutz and his lab are working on multiple fronts to support the need for coronavirus testing. Within days, his team shifted from helping with the Seattle Flu Study to COVID-19, and his lab began developing a community and at-home test.
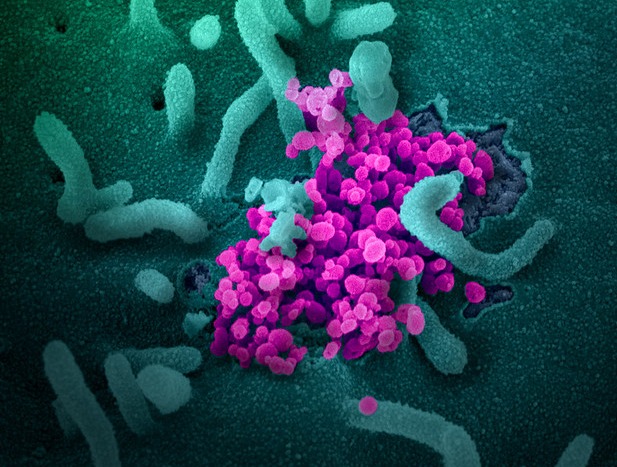
SARS-CoV-2 (purple) emerges from the surface of cells cultured in the lab in this scanning election microscope image. Credit: NIAID
Disease-in-a-dish: studying COVID-19’s impact on organs; testing drugs
Charles Murry, joint professor of pathology, cardiology, and bioengineering and director, ISCRM: The Murry laboratory is leading a collaborative effort with ISCRM investigators and the virology laboratory of Dr. Michael Gale to test whether human heart, kidney and brain organoids can be infected with the SARS-CoV-2 virus. This work already has shown that human heart muscle cells are readily infected, support extensive viral replication, and ultimately are killed by this coronavirus. Their next goals are to use their pioneering “disease-in-a-dish” models to study how the virus affects the simplified organs and do robotically-based, high-throughput screening studies to identify drugs that could prevent the virus from replicating. Nathan Sniadecki, professor of mechanical engineering and adjunct professor in BioE, is a key partner.
 Examining the role of water’s “fourth phase” in coronavirus infection
Examining the role of water’s “fourth phase” in coronavirus infection
Gerald Pollack, professor: Dr. Pollack has been studying the role of water in cell function. His lab identified a “fourth phase” of water that fills biological cells. A decade ago, they found that this phase excludes viruses. They are now considering the possibility that cell water might similarly exclude the novel coronavirus, and if so, whether those who are dehydrated, and therefore deficient in fourth phase water, might be more susceptible than others to the virus.
Credit: CDC
Finding aptamers to bind up the spike protein
Suzie Pun, Robert F. Rushmer Professor: Dr. Pun’s lab is developing a way to block the SARS-CoV-2 spike protein, the major surface protein the virus uses to invade human cells. Dr. Pun received a grant from the Population Health Initiative to identify and develop DNA aptamers, short pieces of DNA, that bind to the spike protein, blocking it from attaching to cells. These aptamers could be used to inhibit infection or be used in diagnostics to sensitively and quickly determine concentrations of intact virus in patient and research samples.
Adapted from Shao, G. et al./ACS Applied Nano Materials 2019.
Developing portable dialysis to meet need for kidney patients at home, in ICU
Buddy Ratner, joint professor of bioengineering and chemical engineering, and Jonathan Himmelfarb, professor of nephrology and adjunct professor of bioengineering, co-directors of the UW Center for Dialysis Innovation (CDI): The CDI team is developing the Ambulatory Kidney to Improve Vitality (AKTIV), a portable kidney dialysis device that works without an external water source. The team’s current goal is to have a first-generation version of the device ready for clinical trials at the end of 2022. To address the COVID-19 pandemic, they propose an accelerated timeline and design changes to meet the pressing need for those with kidney damage. AKTIV technology can be adapted to more safely deliver continuous dialysis in the home and ICU setting with greatly reduced risk of clotting complications and without the complex, water-purification infrastructure needed for today’s dialysis systems. Nature featured CDI’s work in a March news article.
Credit: CDC
Ranking credibility of COVID-19 models
Herbert Sauro, professor: The multi-institution Center for Reproducible Biomedical Modeling, led by Dr. Sauro, is partnering with top U.S. government agencies to determine how credible several commonly used COVID-19 models are.
SARS-CoV-2 particles. Credit: NIAID
Adapting drugamers to inhaled treatment against SARS-CoV-2
Patrick Stayton, Bioengineering Distinguished Term Professor and MolES director: The Stayton lab is adapting a new class of site-specific therapeutic treatments they engineered, called drugamers, that show promise against some of the world’s most dangerous bacteria. The researchers are adapting it to deliver antiviral drugs, and drugs that alter the body’s response, to treat COVID-19 patients. The drugamers would be developed as an inhalable treatment that directly targets key places in the lungs, bypassing the need for IVs and oral drugs. Such a portable inhaler device would reduce nebulizer treatments in crowded and susceptible hospital settings, and offer a key mobile and distributable way to treat patients at home and limit virus transmission.
 Enabling smartphones to take vital signs
Enabling smartphones to take vital signs
Ricky Wang, professor of bioengineering and ophthalmology: The Wang lab has developed a smartphone-based technique that can capture information such as oxygen level, pulse rate, respiratory rate and blood perfusion. They are adapting their low-cost, camera-based optical sensing system to collect vital sign measurements from COVID-19 patients. Symptomatic and asymptomatic people, and health providers could use the system to assess the severity of the illness and help decide if they need hospitalization, reducing unnecessary burden on hospitals. Reassuring numbers would help reduce patient stress. Dr. Wang is the WRF/David and Nancy Auth Innovator of Bioengineering.
SARS-CoV-2 virus particles. Credit: NIAID
Developing rapid COVID-19 tests for the home and clinic
Paul Yager, professor: The Yager lab is applying its rapid, low-cost testing technology, called UbiNAAT, to COVID-19 tests, which could be used by untrained people in their homes as well as in health care facilities and low-resource settings around the world.
Using engineered capillaries model to study COVID-19’s effect on blood vessels
Ying Zheng, associate professor: The Zheng lab has engineered 3D human capillaries to study how blood flows through the microvessels. Now, in collaboration with Dr. Conrad Liles’ allergy and infectious disease laboratory, they plan to test how COVID-19 patient blood serum affects blood vessel permeability using their microvessel model. Their studies may shed light on how to prevent SARS-CoV-2 from promoting blood clots in some patients. The Zheng lab is also working with adjunct professor Dr. Jonathan Himmelfarb and his nephrology lab and plan to study how SARS-CoV2 targets the receptor protein on the surface of cells, called ACE2, to gain cell entry on blood vessels and its impact on kidney tubule functions.



 Developing biomaterials for self-vaccination
Developing biomaterials for self-vaccination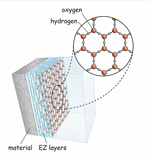 Examining the role of water’s “fourth phase” in coronavirus infection
Examining the role of water’s “fourth phase” in coronavirus infection